White matter changes in dementia: role of impaired drainage of interstitial fluid
- PMID: 25521178
- PMCID: PMC8028907
- DOI: 10.1111/bpa.12218
White matter changes in dementia: role of impaired drainage of interstitial fluid
Abstract
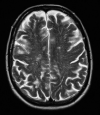
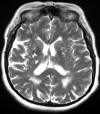
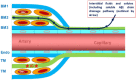
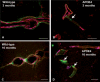
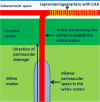
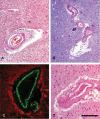
White matter abnormalities on magnetic resonance imaging (MRI) are associated with dementia and include white matter hyperintensities (WMH; also termed leukoaraiosis) and visible perivascular spaces (PVS). We review the potential role of impaired drainage of interstitial fluid in the pathogenesis of WMH and PVS. Whereas the volume of extracellular space in the grey matter is tightly controlled, fluid accumulates and expands the extracellular spaces of the white matter in acute hydrocephalus, vasogenic edema and WMH. Although there are no conventional lymphatic vessels in the brain, there is very effective lymphatic drainage for fluid and solutes along restricted pathways in the basement membranes of cerebral capillaries and arteries in young individuals. Lymphatic drainage of the brain is impaired with age and in association with apolipoprotein E ε4, risk factors for Alzheimer's disease and cerebral amyloid angiopathy (CAA). Deposition of proteins in the lymphatic drainage pathways in the walls of cerebral arteries with age is recognized as protein elimination failure angiopathy (PEFA), as in CAA and cerebral autosomal dominant arteriopathy and leukoencephalopathy (CADASIL). Facilitating perivascular lymphatic drainage from the aging brain may play a significant role in the prevention of CAA, WMH and Alzheimer's disease and may enhance the efficacy of immunotherapy for Alzheimer's disease.
Keywords: Alzheimer's disease; CADASIL; amyloid-β; cerebral amyloid angiopathy; perivascular drainage; perivascular spaces; protein elimination failure angiopathy (PEFA); white matter hyperintensities.
© 2014 International Society of Neuropathology.
Figures




References
-
- Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

