ZEB1 Promotes Invasion in Human Fetal Neural Stem Cells and Hypoxic Glioma Neurospheres
- PMID: 25521330
- PMCID: PMC4470885
- DOI: 10.1111/bpa.12240
ZEB1 Promotes Invasion in Human Fetal Neural Stem Cells and Hypoxic Glioma Neurospheres
Abstract
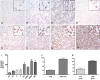
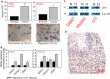
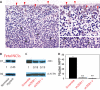
Diffuse spread through brain parenchyma and the presence of hypoxic foci rimmed by neoplastic cells are two cardinal features of glioblastoma, and low oxygen is thought to drive movement of malignant gliomas in the core of the lesions. Transcription factors associated with epithelial-to-mesenchymal transition (EMT) have been linked to this invasion, and we found that hypoxia increased in vitro invasion up to fourfold in glioblastoma neurosphere lines and induced the expression of ZEB1. Immunohistochemical assessment of 295 surgical specimens consisting of various types of pediatric and adult brain cancers showed that ZEB1 expression was significantly higher in infiltrative lesions than less invasive tumors such as pilocytic astrocytoma and ependymoma. ZEB1 protein was also present in human fetal periventricular stem and progenitor cells and ZEB1 inhibition impaired migration of in vitro propagated human neural stem cells. The induction of ZEB1 protein in hypoxic glioblastoma neurospheres could be partially blocked by the HIF1alpha inhibitor digoxin. Targeting ZEB1 blocked hypoxia-augmented invasion of glioblastoma cells in addition to slowing them in normoxia. These data support the role for ZEB1 in invasive and high-grade brain tumors and suggest its key role in promoting invasion in the hypoxic tumor core as well as in the periphery.
Keywords: EMT; ZEB1; glioma; hypoxia; neural stem cell.
© 2014 International Society of Neuropathology.
Figures


Similar articles
-
Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis.Cancer Lett. 2015 Apr 1;359(1):107-16. doi: 10.1016/j.canlet.2015.01.010. Epub 2015 Jan 12. Cancer Lett. 2015. PMID: 25592037
-
SHP-2-upregulated ZEB1 is important for PDGFRα-driven glioma epithelial-mesenchymal transition and invasion in mice and humans.Oncogene. 2016 Oct 27;35(43):5641-5652. doi: 10.1038/onc.2016.100. Epub 2016 Apr 4. Oncogene. 2016. PMID: 27041571 Free PMC article.
-
Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis.Biomed Pharmacother. 2017 Nov;95:711-720. doi: 10.1016/j.biopha.2017.08.133. Epub 2017 Sep 5. Biomed Pharmacother. 2017. PMID: 28886531
-
The roles of hypoxia-inducible factors in regulating neural stem cells migration to glioma stem cells and determinating their fates.Neurochem Res. 2012 Dec;37(12):2659-66. doi: 10.1007/s11064-012-0879-x. Epub 2012 Sep 19. Neurochem Res. 2012. PMID: 22991140 Review.
-
Zinc Finger E-Box Binding Homeobox Family: Non-Coding RNA and Epigenetic Regulation in Gliomas.Biomedicines. 2023 May 5;11(5):1364. doi: 10.3390/biomedicines11051364. Biomedicines. 2023. PMID: 37239035 Free PMC article. Review.
Cited by
-
Robot technology identifies a Parkinsonian therapeutics repurpose to target stem cells of glioblastoma.CNS Oncol. 2020 Jun;9(2):CNS58. doi: 10.2217/cns-2020-0004. Epub 2020 May 28. CNS Oncol. 2020. PMID: 32462934 Free PMC article.
-
CBF1 is clinically prognostic and serves as a target to block cellular invasion and chemoresistance of EMT-like glioblastoma cells.Br J Cancer. 2017 Jun 27;117(1):102-112. doi: 10.1038/bjc.2017.157. Epub 2017 Jun 1. Br J Cancer. 2017. PMID: 28571041 Free PMC article.
-
MicroRNA-200b expression level is negatively associated with pathological grading in human gliomas.Cancer Manag Res. 2018 Aug 24;10:2825-2834. doi: 10.2147/CMAR.S171137. eCollection 2018. Cancer Manag Res. 2018. PMID: 30197535 Free PMC article.
-
MicroRNA-144 regulates proliferation, invasion, and apoptosis of cells in malignant solitary pulmonary nodule via zinc finger E-box-binding homeobox 1.Int J Clin Exp Pathol. 2015 May 1;8(5):5960-7. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191328 Free PMC article.
-
Muscarinic receptor drug trihexyphenidyl can alter growth of mesenchymal glioblastoma in vivo.Front Pharmacol. 2024 Sep 25;15:1468920. doi: 10.3389/fphar.2024.1468920. eCollection 2024. Front Pharmacol. 2024. PMID: 39386028 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

