Selective interaction of heparin with the variable region 3 within surface glycoprotein of laboratory-adapted feline immunodeficiency virus
- PMID: 25521480
- PMCID: PMC4270745
- DOI: 10.1371/journal.pone.0115252
Selective interaction of heparin with the variable region 3 within surface glycoprotein of laboratory-adapted feline immunodeficiency virus
Abstract
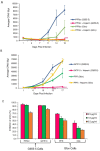
Heparan sulfate proteoglycans (HSPG) can act as binding receptors for certain laboratory-adapted (TCA) strains of feline immunodeficiency virus (FIV) and human immunodeficiency virus (HIV). Heparin, a soluble heparin sulfate (HS), can inhibit TCA HIV and FIV entry mediated by HSPG interaction in vitro. In the present study, we further determined the selective interaction of heparin with the V3 loop of TCA of FIV. Our current results indicate that heparin selectively inhibits infection by TCA strains, but not for field isolates (FS). Heparin also specifically interferes with TCA surface glycoprotein (SU) binding to CXCR4, by interactions with HSPG binding sites on the V3 loop of the FIV envelope protein. Peptides representing either the N- or C-terminal side of the V3 loop and containing HSPG binding sites were able to compete away the heparin block of TCA SU binding to CXCR4. Heparin does not interfere with the interaction of SU with anti-V3 antibodies that target the CXCR4 binding region or with the interaction between FS FIV and anti-V3 antibodies since FS SU has no HSPG binding sites within the HSPG binding region. Our data show that heparin blocks TCA FIV infection or entry not only through its competition of HSPG on the cell surface interaction with SU, but also by its interference with CXCR4 binding to SU. These studies aid in the design and development of heparin derivatives or analogues that can inhibit steps in virus infection and are informative regarding the HSPG/SU interaction.
Conflict of interest statement
Figures
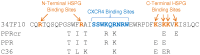
References
-
- Couchman JR, Chen L, Woods A (2001) Syndecans and cell adhesion. Int Rev Cytol 207:113–150. - PubMed
-
- Li JP, Vlodavsky I (2009) Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb Haemost 102:823–828. - PubMed
-
- Dreyfuss JL, Regatieri CV, Jarrouge TR, Cavalheiro RP, Sampaio LO, et al. (2009) Heparan sulfate proteoglycans: structure, protein interactions and cell signaling. An Acad Bras Cienc 81:409–429. - PubMed
-
- Liu D, Shriver Z, Qi Y, Venkataraman G, Sasisekharan R (2002) Dynamic regulation of tumor growth and metastasis by heparan sulfate glycosaminoglycans. Semin Thromb Hemost 28:67–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

