Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues
- PMID: 25522019
- PMCID: PMC4403088
- DOI: 10.1111/bph.13043
Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues
Abstract
Background and purpose: There is growing concern over the abuse of certain psychostimulant methcathinone (MCAT) analogues. This study extends an initial quantitative structure-activity relationship (QSAR) investigation that demonstrated important steric considerations of seven 4- (or para-)substituted analogues of MCAT. Specifically, the steric character (Taft's steric ES ) of the 4-position substituent affected in vitro potency to induce monoamine release via dopamine and 5-HT transporters (DAT and SERT) and in vivo modulation of intracranial self-stimulation (ICSS). Here, we have assessed the effects of other steric properties of the 4-position substituents.
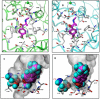
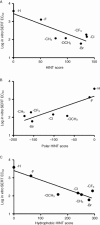
Experimental approach: Definitive steric parameters that more explicitly focus on the volume, width and length of the MCAT 4-position substituents were assessed. In addition, homology models of human DAT and human SERT based upon the crystallized Drosophila DAT were constructed and docking studies were performed, followed by hydropathic interaction (HINT) analysis of the docking results.
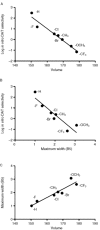
Key results: The potency of seven MCAT analogues at DAT was negatively correlated with the volume and maximal width of their 4-position substituents, whereas potency at SERT increased as substituent volume and length increased. SERT/DAT selectivity, as well as abuse-related drug effects in the ICSS procedure, also correlated with the same parameters. Docking solutions offered a means of visualizing these findings.
Conclusions and implications: These results suggest that steric aspects of the 4-position substituents of MCAT analogues are key determinants of their action and selectivity, and that the hydrophobic nature of these substituents is involved in their potency at SERT.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues.Br J Pharmacol. 2015 May;172(10):2433-44. doi: 10.1111/bph.13030. Epub 2015 Apr 10. Br J Pharmacol. 2015. PMID: 25438806 Free PMC article.
-
Decoding the Structure of Abuse Potential for New Psychoactive Substances: Structure-Activity Relationships for Abuse-Related Effects of 4-Substituted Methcathinone Analogs.Curr Top Behav Neurosci. 2017;32:119-131. doi: 10.1007/7854_2016_18. Curr Top Behav Neurosci. 2017. PMID: 27696217 Free PMC article.
-
para-Trifluoromethyl-methcathinone is an allosteric modulator of the serotonin transporter.Neuropharmacology. 2019 Dec 15;161:107615. doi: 10.1016/j.neuropharm.2019.04.021. Epub 2019 Apr 24. Neuropharmacology. 2019. PMID: 31028773
-
Using Ca2+-channel biosensors to profile amphetamines and cathinones at monoamine transporters: electro-engineering cells to detect potential new psychoactive substances.Psychopharmacology (Berl). 2019 Mar;236(3):973-988. doi: 10.1007/s00213-018-5103-5. Epub 2018 Nov 17. Psychopharmacology (Berl). 2019. PMID: 30448989 Free PMC article. Review.
-
Computational approaches for the study of serotonin and its membrane transporter SERT: implications for drug design in neurological sciences.Curr Med Chem. 2008;15(30):3214-27. doi: 10.2174/092986708786848523. Curr Med Chem. 2008. PMID: 19075665 Review.
Cited by
-
Structure-Activity Relationships of Synthetic Cathinones.Curr Top Behav Neurosci. 2017;32:19-47. doi: 10.1007/7854_2016_41. Curr Top Behav Neurosci. 2017. PMID: 27830576 Free PMC article.
-
Abuse-Related Neurochemical Effects of Para-Substituted Methcathinone Analogs in Rats: Microdialysis Studies of Nucleus Accumbens Dopamine and Serotonin.J Pharmacol Exp Ther. 2016 Jan;356(1):182-90. doi: 10.1124/jpet.115.229559. Epub 2015 Oct 15. J Pharmacol Exp Ther. 2016. PMID: 26645638 Free PMC article.
-
Abuse-related neurochemical and behavioral effects of cathinone and 4-methylcathinone stereoisomers in rats.Eur Neuropsychopharmacol. 2016 Feb;26(2):288-297. doi: 10.1016/j.euroneuro.2015.12.010. Epub 2015 Dec 11. Eur Neuropsychopharmacol. 2016. PMID: 26738428 Free PMC article.
-
Neurotoxicology of Synthetic Cathinone Analogs.Curr Top Behav Neurosci. 2017;32:209-230. doi: 10.1007/7854_2016_21. Curr Top Behav Neurosci. 2017. PMID: 27753008 Free PMC article. Review.
-
Ethylenedioxy homologs of N-methyl-(3,4-methylenedioxyphenyl)-2-aminopropane (MDMA) and its corresponding cathinone analog methylenedioxymethcathinone: Interactions with transporters for serotonin, dopamine, and norepinephrine.Bioorg Med Chem. 2015 Sep 1;23(17):5574-9. doi: 10.1016/j.bmc.2015.07.035. Epub 2015 Jul 23. Bioorg Med Chem. 2015. PMID: 26233799 Free PMC article.
References
-
- Abraham DJ, Kellogg GE. The effect of physical organic properties on hydrophobic fields. J Comput Aided Molec Des. 1994;8:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

