Hepatic arterial spin labelling MRI: an initial evaluation in mice
- PMID: 25522098
- PMCID: PMC4670473
- DOI: 10.1002/nbm.3251
Hepatic arterial spin labelling MRI: an initial evaluation in mice
Abstract
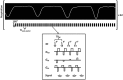
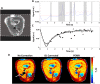
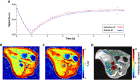
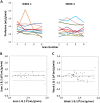
The development of strategies to combat hepatic disease and augment tissue regeneration has created a need for methods to assess regional liver function. Liver perfusion imaging has the potential to fulfil this need, across a range of hepatic diseases, alongside the assessment of therapeutic response. In this study, the feasibility of hepatic arterial spin labelling (HASL) was assessed for the first time in mice at 9.4 T, its variability and repeatability were evaluated, and it was applied to a model of colorectal liver metastasis. Data were acquired using flow-sensitive alternating inversion recovery-arterial spin labelling (FAIR-ASL) with a Look-Locker readout, and analysed using retrospective respiratory gating and a T1 -based quantification. This study shows that preclinical HASL is feasible and exhibits good repeatability and reproducibility. Mean estimated liver perfusion was 2.2 ± 0.8 mL/g/min (mean ± standard error, n = 10), which agrees well with previous measurements using invasive approaches. Estimates of the variation gave a within-session coefficient of variation (CVWS) of 7%, a between-session coefficient of variation (CVBS) of 9% and a between-animal coefficient of variation (CVA) of 15%. The within-session Bland-Altman repeatability coefficient (RCWS) was 18% and the between-session repeatability coefficient (RCBS) was 29%. Finally, the HASL method was applied to a mouse model of liver metastasis, in which significantly lower mean perfusion (1.1 ± 0.5 mL/g/min, n = 6) was measured within the tumours, as seen by fluorescence histology. These data indicate that precise and accurate liver perfusion estimates can be achieved using ASL techniques, and provide a platform for future studies investigating hepatic perfusion in mouse models of disease.
Keywords: ASL; liver; metastasis; mouse; perfusion; preclinical; repeatability; variability.
Copyright © 2014 John Wiley & Sons, Ltd.
Figures
Similar articles
-
Liver perfusion MRI in a rodent model of cirrhosis: Agreement with bulk-flow phase-contrast MRI and noninvasive evaluation of inflammation in chronic liver disease using flow-sensitive alternating inversion recovery arterial spin labelling and tissue T1.NMR Biomed. 2021 Feb;34(2):e4423. doi: 10.1002/nbm.4423. Epub 2020 Oct 7. NMR Biomed. 2021. PMID: 33029872 Free PMC article.
-
Repeatability of renal arterial spin labelling MRI in healthy subjects.MAGMA. 2012 Apr;25(2):145-53. doi: 10.1007/s10334-011-0300-9. Epub 2012 Jan 13. MAGMA. 2012. PMID: 22246289
-
Cardiac arterial spin labeling using segmented ECG-gated Look-Locker FAIR: variability and repeatability in preclinical studies.Magn Reson Med. 2013 Jan;69(1):238-47. doi: 10.1002/mrm.24243. Epub 2012 Mar 12. Magn Reson Med. 2013. PMID: 22411842
-
Arterial spin labelling MRI to measure renal perfusion: a systematic review and statement paper.Nephrol Dial Transplant. 2018 Sep 1;33(suppl_2):ii15-ii21. doi: 10.1093/ndt/gfy180. Nephrol Dial Transplant. 2018. PMID: 30137581 Free PMC article.
-
Advances in arterial spin labelling MRI methods for measuring perfusion and collateral flow.J Cereb Blood Flow Metab. 2018 Sep;38(9):1461-1480. doi: 10.1177/0271678X17713434. Epub 2017 Jun 9. J Cereb Blood Flow Metab. 2018. PMID: 28598243 Free PMC article. Review.
Cited by
-
MRI-based assessment of liver perfusion and hepatocyte injury in the murine model of acute hepatitis.MAGMA. 2016 Dec;29(6):789-798. doi: 10.1007/s10334-016-0563-2. Epub 2016 May 9. MAGMA. 2016. PMID: 27160299 Free PMC article.
-
Acute changes in liver tumour perfusion measured non-invasively with arterial spin labelling.Br J Cancer. 2016 Apr 12;114(8):897-904. doi: 10.1038/bjc.2016.51. Epub 2016 Mar 31. Br J Cancer. 2016. PMID: 27031853 Free PMC article.
-
Organ-specific responses during brain death: increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys.Sci Rep. 2018 Mar 13;8(1):4405. doi: 10.1038/s41598-018-22689-9. Sci Rep. 2018. PMID: 29535334 Free PMC article.
-
Survey of water proton longitudinal relaxation in liver in vivo.MAGMA. 2021 Dec;34(6):779-789. doi: 10.1007/s10334-021-00928-x. Epub 2021 May 12. MAGMA. 2021. PMID: 33978944 Free PMC article. Review.
-
Investigating the Vascular Phenotype of Subcutaneously and Orthotopically Propagated PC3 Prostate Cancer Xenografts Using Combined Carbogen Ultrasmall Superparamagnetic Iron Oxide MRI.Top Magn Reson Imaging. 2016 Oct;25(5):237-243. doi: 10.1097/RMR.0000000000000102. Top Magn Reson Imaging. 2016. PMID: 27748709 Free PMC article. Review.
References
-
- Hoad C, Costigan C, Marciani L, Kaye P, Spiller R, Gowland P, Aithal G, Francis S. Quantifying blood flow and perfusion in liver tissue using phase contrast angiography and arterial spin labelling. Proc. Int. Soc. Magn. Reson. Med. 2011;19:794.
-
- Katada Y, Shukuya T, Kawashima M, Nozaki M, Imai H, Natori T, Tamano M. A comparative study between arterial spin labeling and CT perfusion methods on hepatic portal venous flow. Jpn. J. Radiol. 2012;30(10):863–869. - PubMed
-
- Sarin SK, Sabba C, Groszmann RJ. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am. J. Physiol. 1990;258(3 Pt 1):G365–G369. - PubMed
-
- Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y. Hepatic perfusion parameters in chronic liver disease: dynamic CT measurements correlated with disease severity. Am. J. Roentgenol. 2001;176(3):667–673. - PubMed
-
- Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clement O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218(2):556–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

