Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees
- PMID: 25522974
- PMCID: PMC4324612
- DOI: 10.1016/j.biomaterials.2014.11.035
Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees
Abstract
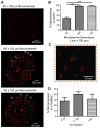
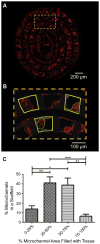
Neurally controlled prosthetics that cosmetically and functionally mimic amputated limbs remain a clinical need because state of the art neural prosthetics only provide a fraction of a natural limb's functionality. Here, we report on the fabrication and capability of polydimethylsiloxane (PDMS) and epoxy-based SU-8 photoresist microchannel scaffolds to serve as viable constructs for peripheral nerve interfacing through in vitro and in vivo studies in a sciatic nerve amputee model where the nerve lacks distal reinnervation targets. These studies showed microchannels with 100 μm × 100 μm cross-sectional areas support and direct the regeneration/migration of axons, Schwann cells, and fibroblasts through the microchannels with space available for future maturation of the axons. Investigation of the nerve in the distal segment, past the scaffold, showed a high degree of organization, adoption of the microchannel architecture forming 'microchannel fascicles', reformation of endoneurial tubes and axon myelination, and a lack of aberrant and unorganized growth that might be characteristic of neuroma formation. Separate chronic terminal in vivo electrophysiology studies utilizing the microchannel scaffolds with permanently integrated microwire electrodes were conducted to evaluate interfacing capabilities. In all devices a variety of spontaneous, sensory evoked and electrically evoked single and multi-unit action potentials were recorded after five months of implantation. Together, these findings suggest that microchannel scaffolds are well suited for chronic implantation and peripheral nerve interfacing to promote organized nerve regeneration that lends itself well to stable interfaces. Thus this study establishes the basis for the advanced fabrication of large-electrode count, wireless microchannel devices that are an important step towards highly functional, bi-directional peripheral nerve interfaces.
Keywords: Microchannel; Nerve guide; Nerve regeneration; Neural interfacing; Peripheral nerve interfacing; Polydimethylsiloxane.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures









References
-
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuro-prostheses and hybrid bionic systems. J Peripher Nerv Syst. 2005;10:229–58. - PubMed
-
- Fitzgerald JJ, Lacour SP, McMahon SB, Fawcett JW. Microchannels as axonal amplifiers. IEEE Trans Biomed Eng. 2008;55:1136–46. - PubMed
-
- Grill WM, Mortimer JT. Neural and connective tissue response to long-term implantation of multiple contact nerve cuff electrodes. J Biomed Mater Res. 2000;50:215–26. - PubMed
-
- Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002;10:294–303. - PubMed
-
- Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51:146–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

