Ventricular septal defect
- PMID: 25523232
- PMCID: PMC4316658
- DOI: 10.1186/s13023-014-0144-2
Ventricular septal defect
Abstract
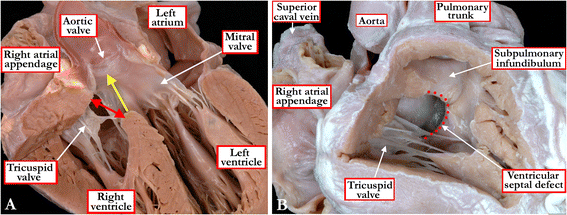
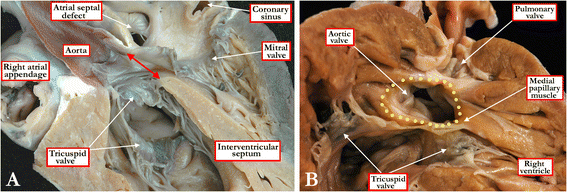
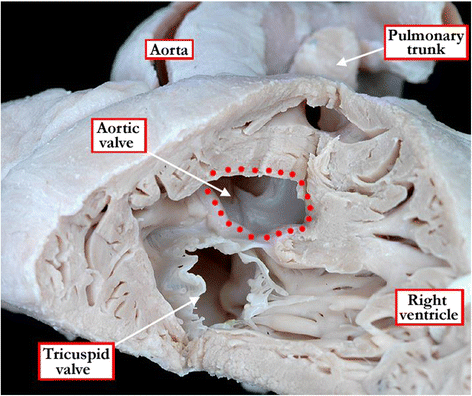
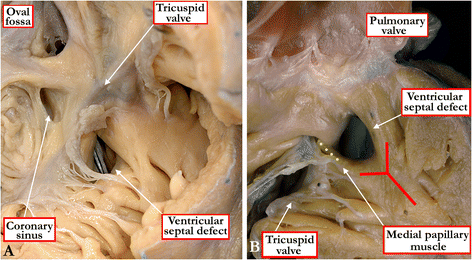
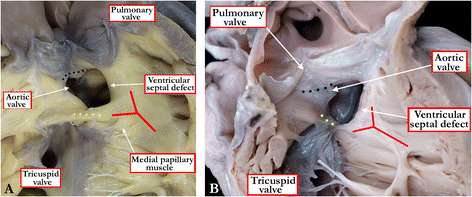
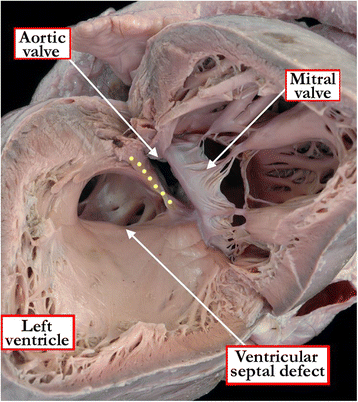
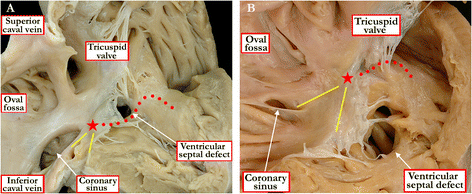
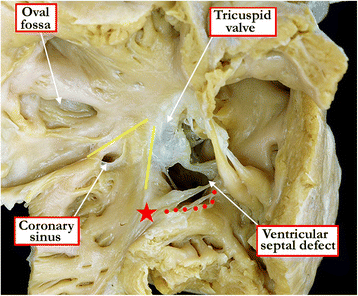
Background: Ventricular septal defects are the commonest congenital cardiac malformations. They can exist in isolation, but are also found as integral components of other cardiac anomalies, such as tetralogy of Fallot, double outlet right ventricle, or common arterial trunk. As yet, there is no agreement on how best to classify such defects, nor even on the curved surface that is taken to represent the defect.
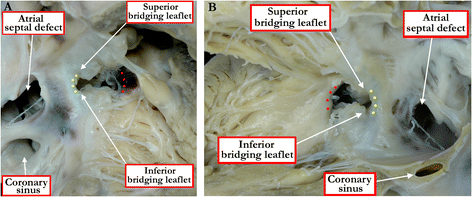
Methods: Based on our previous pathological and clinical experiences, we have reviewed the history of classification of holes between the ventricles. We proposed that the defects are best defined as representing the area of deficient ventricular septation. This then permits the recognition of clinically significant variants according to the anatomic borders, and the way the curved surface representing the area of deficient septation opens into the morphologically right ventricle.
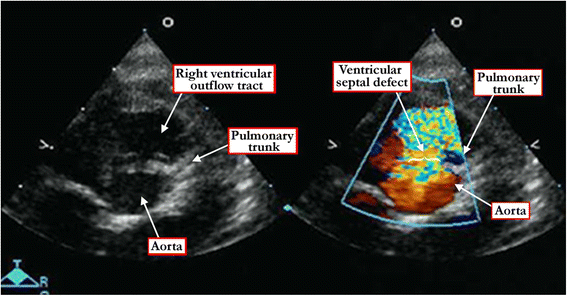
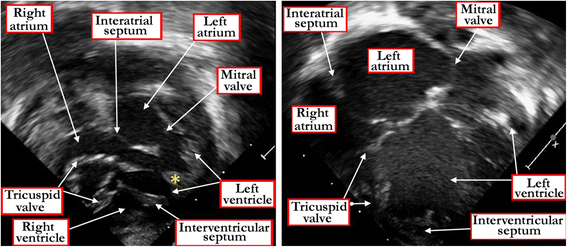
Results: Clinical manifestation depends on the size of the defect, and on the relationship between systemic and pulmonary vascular resistances. Symptoms include failure to thrive, along with the manifestations of the increase in flow of blood to the lungs. Diagnosis can be made by physical examination, but is confirmed by echocardiographic interrogation, which delineates the precise anatomy, and also provides the physiologic information required for optimal clinical decision-making. Cardiac catheterization offers additional information regarding hemodynamics, particularly if there is a concern regarding an increase in pulmonary vascular resistance. Hemodynamic assessment is rarely necessary to make decisions regarding management, although it can be helpful if assessing symptomatic adults with hemodynamically restrictive defects. In infants with defects producing large shunts, surgical closure is now recommended in most instances as soon as symptoms manifest. Only in rare cases is palliative banding of the pulmonary trunk now recommended. Closure with devices inserted on catheters is now the preferred approach for many patients with muscular defects, often using a hybrid procedure. Therapeutic closure should now be anticipated with virtually zero mortality, and with excellent anticipated long-term survival.
Conclusion: Ventricular septal defects are best defined as representing the borders of the area of deficient ventricular septation. An approach on this basis permits recognition of the clinically significant phenotypic variants.
Figures




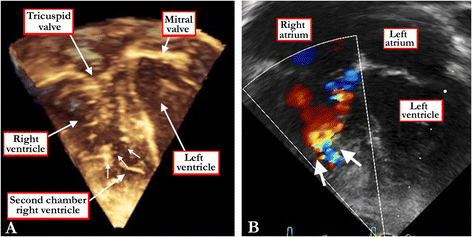
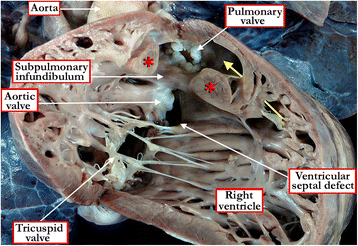
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

