Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice
- PMID: 25523827
- PMCID: PMC4297430
- DOI: 10.1186/1750-1326-9-58
Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice
Abstract
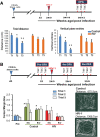
Background: Host-species specificity of the human immunodeficiency virus (HIV) limits pathobiologic, diagnostic and therapeutic research investigations to humans and non-human primates. The emergence of humanized mice as a model for viral infection of the nervous system has overcome such restrictions enabling research for HIV-associated end organ disease including behavioral, cognitive and neuropathologic deficits reflective of neuroAIDS. Chronic HIV-1 infection of NOD/scid-IL-2Rgcnull mice transplanted with human CD34+ hematopoietic stem cells (CD34-NSG) leads to persistent viremia, profound CD4+ T lymphocyte loss and infection of human monocyte-macrophages in the meninges and perivascular spaces. Murine cells are not infected with virus.
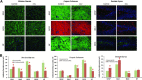
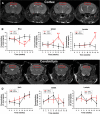
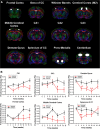
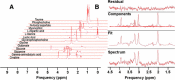
Methods: Changes in mouse behavior were measured, starting at 8 weeks after viral infection. These were recorded coordinate with magnetic resonance spectroscopy metabolites including N-acetylaspartate (NAA), creatine and choline. Diffusion tensor magnetic resonance imaging (DTI) was recorded against multispectral immunohistochemical staining for neuronal markers that included microtubule associated protein-2 (MAP2), neurofilament (NF) and synaptophysin (SYN); for astrocyte glial fibrillary acidic protein (GFAP); and for microglial ionized calcium binding adaptor molecule 1 (Iba-1). Oligodendrocyte numbers and integrity were measured for myelin associated glycoprotein (MAG) and myelin oligodendrocyte glycoprotein (MOG) antigens.
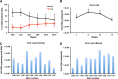
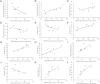
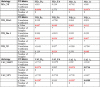
Results: Behavioral abnormalities were readily observed in HIV-1 infected mice. Longitudinal open field activity tests demonstrated lack of habituation indicating potential for memory loss and persistent anxiety in HIV-1 infected mice compared to uninfected controls. End-point NAA and creatine in the cerebral cortex increased with decreased MAG. NAA and glutamate decreased with decreased SYN and MAG. Robust inflammation reflected GFAP and Iba-1 staining intensities. DTI metrics were coordinate with deregulation of NF, Iba-1, MOG and MAG levels in the whisker barrel and MAP2, NF, MAG, MOG and SYN in the corpus callosum.
Conclusions: The findings are consistent with some of the clinical, biochemical and pathobiologic features of human HIV-1 nervous system infections. This model will prove useful towards investigating the mechanisms of HIV-1 induced neuropathology and in developing novel biomarkers and therapeutic strategies for disease.
Figures


References
-
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. - DOI - PMC - PubMed
-
- Pozniak A, Rackstraw S, Deayton J, Barber T, Taylor S, Manji H, Melvin D, Croston M, Nightingale S, Kulasegaram R, Pitkanen M, Winston A. HIV-associated neurocognitive disease: case studies and suggestions for diagnosis and management in different patient sub-groups. Antivir Ther. 2014;19(1):1–13. doi: 10.3851/IMP2563. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 NS31492/NS/NINDS NIH HHS/United States
- 2R01 NS034239/NS/NINDS NIH HHS/United States
- P01 MH64570/MH/NIMH NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 MH104147/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- P01 NS43985/NS/NINDS NIH HHS/United States
- R24 OD018546/OD/NIH HHS/United States
- R01 NS36126/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

