Role of Fn14 in acute alcoholic steatohepatitis in mice
- PMID: 25524063
- PMCID: PMC4329478
- DOI: 10.1152/ajpgi.00429.2013
Role of Fn14 in acute alcoholic steatohepatitis in mice
Abstract
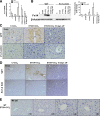
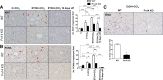
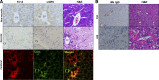
TNF-like weak inducer of apoptosis (TWEAK) is a growth factor for bipotent liver progenitors that express its receptor, fibroblast growth factor-inducible 14 (Fn14), a TNF receptor superfamily member. Accumulation of Fn14(+) progenitors occurs in severe acute alcoholic steatohepatitis (ASH) and correlates with acute mortality. In patients with severe ASH, inhibition of TNF-α increases acute mortality. The aim of this study was to determine whether deletion of Fn14 improves the outcome of liver injury in alcohol-consuming mice. Wild-type (WT) and Fn14 knockout (KO) mice were fed control high-fat Lieber deCarli diet or high-fat Lieber deCarli diet with 2% alcohol (ETOH) and injected intraperitoneally with CCl₄ for 2 wk to induce liver injury. Mice were euthanized 3 or 10 days after CCl₄ treatment. Survival was assessed. Liver tissues were analyzed for cell death, inflammation, proliferation, progenitor accumulation, and fibrosis by quantitative RT-PCR, immunoblot, hydroxyproline content, and quantitative immunohistochemistry. During liver injury, Fn14 expression, apoptosis, inflammation, hepatocyte replication, progenitor and myofibroblast accumulation, and fibrosis increased in WT mice fed either diet. Mice fed either diet expressed similar TWEAK/Fn14 levels, but ETOH-fed mice had higher TNF-α expression. The ETOH-fed group developed more apoptosis, inflammation, fibrosis, and regenerative responses. Fn14 deletion did not reduce hepatic TNF-α expression but improved all injury parameters in mice fed the control diet. In ETOH-fed mice, Fn14 deletion inhibited TNF-α induction and increased acute mortality, despite improvement in liver injury. Fn14 mediates wound-healing responses that are necessary to survive acute liver injury during alcohol exposure.
Keywords: alcohol; liver fibrosis; liver injury; liver progenitors.
Figures







References
-
- Affo S, Dominguez M, Lozano JJ, Sancho-Bru P, Rodrigo-Torres D, Morales-Ibanez O, Moreno M, Millan C, Loaeza-del-Castillo A, Altamirano J, Garcia-Pagan JC, Arroyo V, Gines P, Caballeria J, Schwabe RF, Bataller R. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 62: 452–460, 2013. - PMC - PubMed
-
- An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol 86: 1337–1348, 2012. - PubMed
-
- Bird TG, Lu WY, Boulter L, Gordon-Keylock S, Ridgway RA, Williams MJ, Taube J, Thomas JA, Wojtacha D, Gambardella A, Sansom OJ, Iredale JP, Forbes SJ. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA 110: 6542–6547, 2013. - PMC - PubMed
-
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, Sansom OJ, Iredale JP, Lowell S, Roskams T, Forbes SJ. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579, 2012. - PMC - PubMed
-
- Diehl AM, Yin M, Fleckenstein J, Yang SQ, Lin HZ, Brenner DA, Westwick J, Bagby G, Nelson S. Tumor necrosis factor-α induces c-jun during the regenerative response to liver injury. Am J Physiol Gastrointest Liver Physiol 267: G552–G561, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

