Molecular controls of lymphatic VEGFR3 signaling
- PMID: 25524775
- PMCID: PMC4304921
- DOI: 10.1161/ATVBAHA.114.304881
Molecular controls of lymphatic VEGFR3 signaling
Abstract
Objectives: Vascular endothelial growth factor receptor 3 (VEGFR3) plays important roles both in lymphangiogenesis and angiogenesis. On stimulation by its ligand VEGF-C, VEGFR3 is able to form both homodimers as well as heterodimers with VEGFR2 and activates several downstream signal pathways, including extracellular signal-regulated kinases (ERK)1/2 and protein kinase B (AKT). Despite certain similarities with VEGFR2, molecular features of VEGFR3 signaling are still largely unknown.
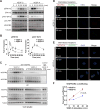
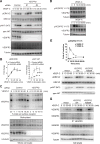
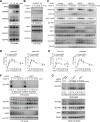
Approach and results: Human dermal lymphatic endothelial cells were used to examine VEGF-C-driven activation of signaling. Compared with VEGF-A activation of VEGFR2, VEGF-C-induced VEGFR3 activation led to a more extensive AKT activation, whereas activation of ERK1/2 displayed a distinctly different kinetics. Furthermore, VEGF-C, but not VEGF-A, induced formation of VEGFR3/VEGFR2 complexes. Silencing VEGFR2 or its partner neuropilin 1 specifically abolished VEGF-C-induced AKT but not ERK activation, whereas silencing of neuropilin 2 had little effect on either signaling pathway. Finally, suppression of vascular endothelial phosphotyrosine phosphatase but not other phosphotyrosine phosphatases enhanced VEGF-C-induced activation of both ERK and AKT pathways. Functionally, both ERK and AKT pathways are important for lymphatic endothelial cells migration.
Conclusions: VEGF-C activates AKT signaling via formation of VEGFR3/VEGFR2 complex, whereas ERK is activated by VEGFR3 homodimer. Neuropilin 1 and vascular endothelial phosphotyrosine phosphatase are involved in regulation of VEGFR3 signaling.
Keywords: NRP1; NRP2; VE-PTP; VEGF-C; VEGFR2; VEGFR3.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Disclosure: The authors have declared that no conflict of interest exists.
Figures


References
-
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. - PubMed
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. - PubMed
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, et al. Notch-dependent vegfr3 upregulation allows angiogenesis without vegf-vegfr2 signalling. Nature. 2012;484:110–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

