Pioglitazone restores IGFBP-3 levels through DNA PK in retinal endothelial cells cultured in hyperglycemic conditions
- PMID: 25525174
- PMCID: PMC4294286
- DOI: 10.1167/iovs.14-15550
Pioglitazone restores IGFBP-3 levels through DNA PK in retinal endothelial cells cultured in hyperglycemic conditions
Abstract
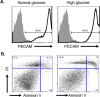
Purpose: Previously, we reported that pioglitazone prevented insulin resistance and cell death in type 2 diabetic retina by reducing TNFα and suppressor of cytokine signaling 3 (SOCS3) levels. Numerous reports suggest prominent vasoprotective effects of insulin growth factor binding protein-3 (IGFBP-3) in diabetic retinopathy. We hypothesized that pioglitazone protects against retinal cell apoptosis by regulating IGFBP-3 levels, in addition to reducing TNFα. The current study explored potential IGFBP-3 regulatory pathways by pioglitazone in retinal endothelial cells cultured in high glucose.
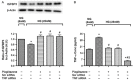
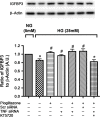
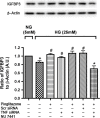
Methods: Primary human retinal endothelial cells (REC) were grown in normal (5 mM) and high glucose (25 mM) and treated with pioglitazone for 24 hours. Cell lysates were processed for Western blotting and ELISA analysis to evaluate IGFBP-3, TNFα, and cleaved caspase 3 protein levels.
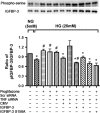
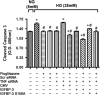
Results: Our results show that treatment with pioglitazone restored the high glucose-induced decrease in IGFBP-3 levels. This regulation was independent of TNFα actions, as reducing TNFα levels with siRNA did not prevent pioglitazone from increasing IGFBP-3 levels. Pioglitazone required protein kinase A (PKA) and DNA-dependent protein kinase (DNA PK) activity to regulate IGFBP-3, as specific inhibitors for each protein prevented pioglitazone-mediated normalization of IGFBP-3 in high glucose. Insulin growth factor binding protein-3 activity was increased and apoptosis decreased by pioglitazone, which was eliminated when serine site 156 of IGFBP-3 was mutated suggesting a key role of this phosphorylation site in pioglitazone actions.
Conclusions: Our findings suggest that pioglitazone mediates regulation of IGFBP-3 via activation of PKA/DNA PK pathway in hyperglycemic retinal endothelial cells.
Keywords: DNA PK; IGFBP-3; pioglitazone; retinal endothelial cells.
Copyright 2015 The Association for Research in Vision and Ophthalmology, Inc.
Figures
References
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010; 376: 124–136. - PubMed
-
- Tawfik A, Sanders T, Kahook K, Akeel S, Elmarakby A, Al-Shabrawey M. Suppression of retinal peroxisome proliferator-activated receptor gamma in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Invest Ophthalmol Vis Sci. 2009; 50: 878–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

