Bacteriophage SPP1 tail tube protein self-assembles into β-structure-rich tubes
- PMID: 25525268
- PMCID: PMC4319047
- DOI: 10.1074/jbc.M114.613166
Bacteriophage SPP1 tail tube protein self-assembles into β-structure-rich tubes
Abstract
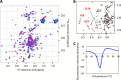
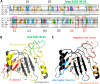
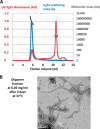
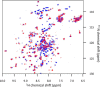
The majority of known bacteriophages have long tails that serve for bacterial target recognition and viral DNA delivery into the host. These structures form a tube from the viral capsid to the bacterial cell. The tube is formed primarily by a helical array of tail tube protein (TTP) subunits. In phages with a contractile tail, the TTP tube is surrounded by a sheath structure. Here, we report the first evidence that a phage TTP, gp17.1 of siphophage SPP1, self-assembles into long tubes in the absence of other viral proteins. gp17.1 does not exhibit a stable globular structure when monomeric in solution, even if it was confidently predicted to adopt the β-sandwich fold of phage λ TTP. However, Fourier transform infrared and nuclear magnetic resonance spectroscopy analyses showed that its β-sheet content increases significantly during tube assembly, suggesting that gp17.1 acquires a stable β-sandwich fold only after self-assembly. EM analyses revealed that the tube is formed by hexameric rings stacked helicoidally with the same organization and helical parameters found for the tail of SPP1 virions. These parameters were used to build a pseudo-atomic model of the TTP tube. The large loop spanning residues 40-56 is located on the inner surface of the tube, at the interface between adjacent monomers and hexamers. In line with our structural predictions, deletion of this loop hinders gp17.1 tube assembly in vitro and interferes with SPP1 tail assembly during phage particle morphogenesis in bacteria.
Keywords: Bacteriophage; Electron Microscopy; Fourier Transform IR (FTIR); Solid State NMR; Tail Tube; Tertiary Structure; Virion; Virus Assembly.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Ackermann H. W. (2007) 5500 phages examined in the electron microscope. Arch. Virol. 152, 227–243 - PubMed
-
- Katsura I. (1990) Mechanism of length determination in bacteriophage λ tails. Adv. Biophys. 26, 1–18 - PubMed
-
- King J. (1971) Bacteriophage T4 tail assembly: four steps in core formation. J. Mol. Biol. 58, 693–709 - PubMed
-
- Davidson A. R., Cardarelli L., Pell L. G., Radford D. R., Maxwell K. L. (2012) Long noncontractile tail machines of bacteriophages. Adv. Exp. Med. Biol. 726, 115–142 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

