Native T1 mapping of the heart - a pictorial review
- PMID: 25525401
- PMCID: PMC4251189
- DOI: 10.4137/CMC.S19005
Native T1 mapping of the heart - a pictorial review
Abstract
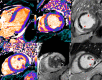
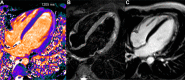
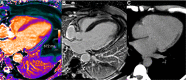
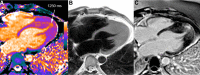
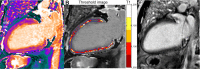
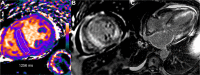
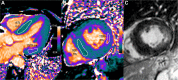
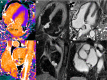
T1 mapping is now a clinically feasible method, providing pixel-wise quantification of the cardiac structure's T1 values. Beyond focal lesions, well depicted by late gadolinium enhancement sequences, it has become possible to discriminate diffuse myocardial alterations, previously not assessable by noninvasive means. The strength of this method includes the high reproducibility and immediate clinical applicability, even without the use of contrast media injection (native or pre-contrast T1). The two most important determinants of native T1 augmentation are (1) edema related to tissue water increase (recent infarction or inflammation) and (2) interstitial space increase related to fibrosis (infarction scar, cardiomyopathy) or to amyloidosis. Conversely, lipid (Anderson-Fabry) or iron overload diseases are responsible for T1 reduction. In this pictorial review, the main features provided by native T1 mapping are discussed and illustrated, with a special focus on the awaited clinical purpose of this unique, promising new method.
Keywords: T1 mapping; cardiac magnetic resonance; cardiomyopathy; edema; fibrosis; myocardial infarction.
Figures






References
-
- Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004;52(1):141–6. - PubMed
-
- Nagel E, Narula J. Evolution and revolution in CMR imaging. JACC Cardiovasc Imaging. 2013;6(7):837–8. - PubMed
-
- Higgins CB, Herfkens R, Lipton MJ, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52(1):184–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

