Cerebral hypoperfusion-assisted intra-arterial deposition of liposomes in normal and glioma-bearing rats
- PMID: 25525695
- PMCID: PMC4273869
- DOI: 10.1227/NEU.0000000000000552
Cerebral hypoperfusion-assisted intra-arterial deposition of liposomes in normal and glioma-bearing rats
Abstract
Background: Optimizing liposomal vehicles for targeted delivery to the brain has important implications for the treatment of brain tumors. The promise of efficient, brain-specific delivery of chemotherapeutic compounds via liposomal vehicles has yet to be achieved in clinical practice. Intra-arterial injection of specially designed liposomes may facilitate efficient delivery to the brain and to gliomas.
Objective: To test the hypothesis that cationic liposomes may be effectively delivered to both normal and glioma-bearing brain tissue utilizing a strategy of intra-arterial injection during transient cerebral hypoperfusion.
Methods: Cationic, anionic, and neutral liposomes were separately injected via the internal carotid artery of healthy rats during transient cerebral hypoperfusion. Rats bearing C6 gliomas were similarly injected with cationic liposomes. Liposomes were loaded with DilC18(5) dye whose concentrations can be measured by light absorbance and fluorescence methods.
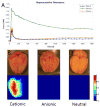
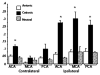
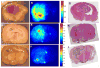
Results: After intra-arterial injection, a robust uptake of cationic in comparison with anionic and neutral liposomes into brain parenchyma was observed by diffuse reflectance spectroscopy. Postmortem multispectral fluorescence imaging revealed that liposomal cationic charge was associated with more efficient delivery to the brain. Cationic liposomes were also readily observed within glioma tissue after intra-arterial injection. However, over time, cationic liposomes were retained longer and at higher concentrations in the surrounding, peritumoral brain than in the tumor core.
Conclusion: This study demonstrates the feasibility of cationic liposome delivery to brain and glioma tissue after intra-arterial injection. Highly cationic liposomes directly delivered to the brain via an intracarotid route may represent an effective method for delivering antiglioma agents.
Figures



References
-
- Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008 Mar 19; - PubMed
-
- Straubinger RM, Arnold RD, Zhou R, Mazurchuk R, Slack JE. Antivascular and antitumor activities of liposome-associated drugs. Anticancer Res. 2004 Mar-Apr;24(2A):397–404. - PubMed
-
- Oldfield EH, Dedrick RL, Yeager RL, et al. Reduced systemic drug exposure by combining intra-arterial chemotherapy with hemoperfusion of regional venous drainage. J Neurosurg. 1985;63(5):726–732. - PubMed
-
- Dedrick RL. Arterial drug infusion: pharmacokinetic problems and pitfalls. Journal of the National Cancer Institute. 1988;80(2):84–89. - PubMed
-
- Riina HA, Burkhardt JK, Santillan A, Bassani L, Patsalides A, Boockvar JA. Short-term clinico-radiographic response to super-selective intra-arterial cerebral infusion of Bevacizumab for the treatment of vestibular schwannomas in Neurofibromatosis type 2. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2012 Jun;18(2):127–132. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

