A sensitive A3B porphyrin nanomaterial for CO2 detection
- PMID: 25525824
- PMCID: PMC6271838
- DOI: 10.3390/molecules191221239
A sensitive A3B porphyrin nanomaterial for CO2 detection
Abstract
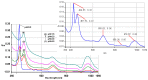
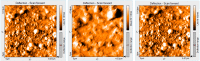
The present report deals with the tailoring, preparation and characterization of novel nanomaterials sensitive to CO2 for use in detection of this gas during space habitation missions. A new nanostructured material based on mixed substituted asymmetrical A3B porphyrin: 5-(4-pyridyl)-10,15,20-tris(3,4-dimethoxyphenyl)-porphyrin (PyTDMeOPP) was synthesized and characterized by 1H-NMR, FT-IR, UV-vis, fluorescence, MS, HPLC and AFM. Introducing one pyridyl substituent in the 5-meso-position of porphyrin macrocycle confers some degree of hydrophilicity, which may cause self-assembly properties and a better response to increased acidity. The influence of pH and nature of the solvent upon H and J aggregates of the porphyrin are discussed. Porphyrin aggregation at the air-THF interface gave a triangular type morphology, randomly distributed but uniformly oriented. When deposition was made by multiple drop-casting operations, a network of triangles of uniform size was created and a porous structure was obtained, being reorganized finally in rings. When the deposition was made from CHCl3, ring structures ranging in internal diameter from 300 nm to 1 µm, but with the same width of the corona circular of approx. 200 nm were obtained. This porphyrin-based material, capable of generating ring aggregates in both THF and CHCl3, has been proven to be sensitive to CO2 detection. The dependence between the intensity of porphyrin UV-vis absorption and the concentration of CO2 has a good correlation of 98.4%.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Amao Y., Nakamura N. Optical CO2 sensor with the combination of colorimetric change of α-naphtholphthalein and internal reference fluorescent porphyrin dye. Sens. Actuators B. 2004;100:347–351. doi: 10.1016/j.snb.2004.02.003. - DOI
-
- Amao Y., Komori T., Nishide H. Rapid responsible optical CO2 sensor of the combination of colorimetric change of α-naphtholphthalein in poly(trimethylsiliylpropyne) layer and internal reference fluorescent porphyrin in polystyrene layer. React. Funct. Polym. 2005;63:35–41. doi: 10.1016/j.reactfunctpolym.2005.02.003. - DOI - PubMed
-
- Amao Y., Nakamura N. An optical sensor with the combination of colorimetric change of α-naphtholphthalein and internal reference luminescent dye for CO2 in water. Sens. Actuators B. 2005;107:861–865. doi: 10.1016/j.snb.2004.12.028. - DOI
-
- Borchert N.B., Kerry J.P., Papkovsky D.B. A CO2 sensor based on Pt-porphyrin dye and FRET scheme for food packaging applications. Sens. Actuators B. 2013;176:157–165. doi: 10.1016/j.snb.2012.09.043. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

