Acetate is a bioenergetic substrate for human glioblastoma and brain metastases
- PMID: 25525878
- PMCID: PMC4374602
- DOI: 10.1016/j.cell.2014.11.025
Acetate is a bioenergetic substrate for human glioblastoma and brain metastases
Abstract
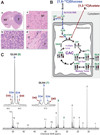
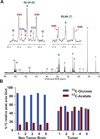
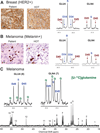
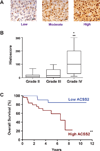
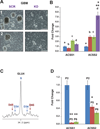
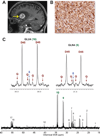
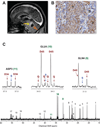
Glioblastomas and brain metastases are highly proliferative brain tumors with short survival times. Previously, using (13)C-NMR analysis of brain tumors resected from patients during infusion of (13)C-glucose, we demonstrated that there is robust oxidation of glucose in the citric acid cycle, yet glucose contributes less than 50% of the carbons to the acetyl-CoA pool. Here, we show that primary and metastatic mouse orthotopic brain tumors have the capacity to oxidize [1,2-(13)C]acetate and can do so while simultaneously oxidizing [1,6-(13)C]glucose. The tumors do not oxidize [U-(13)C]glutamine. In vivo oxidation of [1,2-(13)C]acetate was validated in brain tumor patients and was correlated with expression of acetyl-CoA synthetase enzyme 2, ACSS2. Together, the data demonstrate a strikingly common metabolic phenotype in diverse brain tumors that includes the ability to oxidize acetate in the citric acid cycle. This adaptation may be important for meeting the high biosynthetic and bioenergetic demands of malignant growth.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

