Antibiotic regimens for management of intra-amniotic infection
- PMID: 25526426
- PMCID: PMC10562955
- DOI: 10.1002/14651858.CD010976.pub2
Antibiotic regimens for management of intra-amniotic infection
Abstract
Background: Chorioamnionitis is a common infection that affects both mother and infant. Infant complications associated with chorioamnionitis include early neonatal sepsis, pneumonia, and meningitis. Chorioamnionitis can also result in maternal morbidity such as pelvic infection and septic shock.Clinical chorioamnionitis is estimated to occur in 1% to 2% of term births and in 5% to 10% of preterm births; histologic chorioamnionitis is found in nearly 20% of term births and in 50% of preterm births. Women with chorioamnionitis have a two to three times higher risk for cesarean delivery and a three to four times greater risk for endomyometritis, wound infection, pelvic abscess, bacteremia, and postpartum hemorrhage.
Objectives: To assess the effects of administering antibiotic regimens for intra-amniotic infection on maternal and perinatal morbidity and mortality and on infection-related complications.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 October 2014), CENTRAL, MEDLINE, Embase, LILACS, and the WHO ICTRP (September 2014). We also searched reference lists of retrieved studies and contacted experts in the field.
Selection criteria: Randomized controlled trials (RCTs) that included women who experienced intra-amniotic infection. Trials were included if they compared antibiotic treatment with placebo or no treatment (if applicable), treatment with different antibiotic regimens, or timing of antibiotic therapy (intrapartum and/or postpartum). Therefore, this review assesses trials evaluating intrapartum antibiotics, intrapartum and postpartum antibiotic regimens, and postpartum antibiotics. Diagnosis of intra-amniotic infection was based on standard criteria (clinical/test), and no limit was placed on gestational age.
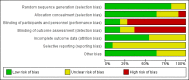
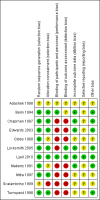
Data collection and analysis: Two review authors independently assessed trials for inclusion and trial quality. Two review authors independently extracted data and checked them for accuracy. We assessed the quality of the evidence using the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach and included a 'Summary of findings' table.
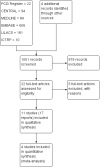
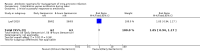
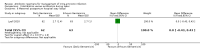
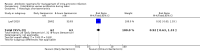
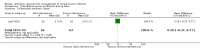
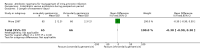
Main results: Our prespecified primary outcomes were maternal and neonatal mortality, maternal and neonatal severe infection, and duration of maternal and neonatal hospital stay.We included 11 studies (involving 1296 women) and assessed them as having low to moderate risk of bias - mainly because allocation concealment methods were not adequately reported, most studies were open, and outcome reporting was incomplete. The quality of the evidence was low to very low for most outcomes, as per the GRADE approach. The following antibiotics were assessed in the included trials: ampicillin, ampicillin/sulbactam, gentamicin, clindamycin, and cefotetan. During labor: meta-analysis of two studies found no clear differences in rates of neonatal sepsis (163 neonates; risk ratio (RR) 1.07, 95% confidence interval (CI) 0.40 to 2.86; I² = 9%; low quality of evidence), treatment failure (endometritis) (163 participants; RR 0.86, 95% CI 0.27 to 2.70; I² = 0%; low quality of evidence), and postpartum hemorrhage (RR 1.39, 95% CI 0.76 to 2.56; I² = 0%; low quality of evidence) when two different dosages/regimens of gentamicin were assessed. No clear differences between groups were found for any reported maternal or neonatal outcomes. The review did not identify data for a comparison of antibiotics versus no treatment/placebo. Postpartum: meta-analysis of two studies that evaluated use of antibiotics versus placebo after vaginal delivery showed no significant differences between groups in rates of treatment failure or postpartum endometritis. No significant differences were found in rates of neonatal death and postpartum endometritis when use of antibiotics was compared with no treatment. Four trials assessing two different dosages/regimens of gentamicin or dual-agent therapy versus triple-agent therapy, or comparing antibiotics, found no significant differences in most reported neonatal or maternal outcomes; the duration of hospital stay showed a difference in favor of the group of women who received short-duration antibiotics (one study, 292 women; mean difference (MD) -0.90 days, 95% CI -1.64 to -0.16; moderate quality of evidence). Intrapartum versus postpartum: one small study (45 women) evaluating use of ampicillin/gentamicin during intrapartum versus immediate postpartum treatment found significant differences favoring the intrapartum group in the mean number of days of maternal postpartum hospital stay (one trial, 45 women; MD -1.00 days, 95% CI -1.94 to - 0.06; very low quality of evidence) and the mean number of neonatal hospital stay days (one trial, 45 neonates; MD -1.90 days, 95% CI -3.91 to -0.49; very low quality of evidence). Although no significant differences were found in the rate of maternal bacteremia or early neonatal sepsis, for the outcome of neonatal pneumonia or sepsis we observed a significant difference favoring intrapartum treatment (one trial, 45 neonates; RR 0.06, 95% CI 0.00 to 0.95; very low quality of evidence).
Authors' conclusions: This review included 11 studies (having low to moderate risk of bias). The quality of the evidence was low to very low for most outcomes, as per the GRADE approach. Only one outcome (duration of hospital stay) was considered to provide moderate quality of evidence when antibiotics (short duration) were compared with antibiotics (long duration) during postpartum management of intra-amniotic infection. Our main reasons for downgrading the quality of evidence were limitations in study design or execution (risk of bias), imprecision, and inconsistency of results.Currently, limited evidence is available to reveal the most appropriate antimicrobial regimen for the treatment of patients with intra-amniotic infection; whether antibiotics should be continued during the postpartum period; and which antibiotic regimen or what treatment duration should be used. Also, no evidence was found on adverse effects of the intervention (not reported in any of the included studies). One small RCT showed that use of antibiotics during the intrapartum period is superior to their use during the postpartum period in reducing the number of days of maternal and neonatal hospital stay.
Conflict of interest statement
None known.
Figures









































Update of
References
References to studies included in this review
Adashek 1998 {published data only}
-
- Adashek JA, Asarat T, Lagrew DC, Rumney PJ, Adhoot D, Iriye BK, et al. The effect of antibiotics after a vaginal delivery complicated by chorioamnionitis in preventing the development of postpartum endomyometritis: a prospectively randomized double‐blind placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S212.
Berry 1994 {published data only}
-
- Berry C, Hansen KA, McCaul JF. Abbreviated antibiotic therapy for clinical chorioamnionitis: a randomized trial. Journal of Maternal‐Fetal Medicine 1994;3(5):216‐8.
-
- Berry C, Hansen KA, McCaul JF. Single dose antibiotic therapy for clinical chorioamnionitis prior to vaginal delivery. American Journal of Obstetrics and Gynecology 1992;166:441.
Chapman 1997 {published data only}
-
- Chapman S, Owen J. Randomized trial of single dose versus multiple dose cefotetan for the postpartum treatment of intrapartum chorioamnionitis. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S59. - PubMed
-
- Chapman SJ, Owen J. Randomized trial of single‐dose versus multiple‐dose cefotetan for the postpartum treatment of intrapartum chorioamnionitis. American Journal of Obstetrics and Gynecology 1997;177(4):831‐4. - PubMed
Edwards 2003 {published data only}
-
- Edwards RK, Duff P. One additional dose of antibiotics is sufficient postpartum therapy for chorioamnionitis [abstract]. Infectious Diseases in Obstetrics and Gynecology 2004;12(3/4):167.
-
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstetrics & Gynecology 2003;102(5 Pt 1):957‐61. - PubMed
Gibbs 1988 {published data only}
-
- Gibbs RS, Dinsmoor MJ, Newton ER, Ramamurthy RS. A randomized trial of intrapartum versus immediate postpartum treatment of women with intra‐amniotic infection. Obstetrics & Gynecology 1988;72:823‐8. - PubMed
Locksmith 2005 {published data only}
-
- Locksmith G, Chin A, Vu T, Shattuck K, Hankins G. High versus standard dosing of gentamicin in women with chorioamnionitis: a randomized, controlled, pharmacokinetic analysis [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S103.
-
- Locksmith GJ, Chin A, Vu T, Shattuck KE, Hankins GDV. High compared with standard gentamicin dosing for chorioamnionitis: a comparison of maternal and fetal serum drug levels. Obstetrics & Gynecology 2005;105:473‐9. - PubMed
Lyell 2010 {published data only}
-
- Lyell DJ, Pullen K, Fuh K, Zamah AM, Caughey AB, Benitz W, et al. Daily compared with 8‐hour gentamicin for the treatment of intrapartum chorioamnionitis: a randomized controlled trial. Obstetrics & Gynecology 2010;115(2 Pt 1):344‐9. - PubMed
-
- Pullen K, Zamah M, Fuh K, Caughey A, Benitz W, Lyell D, et al. Once daily vs. 8 hour gentamicin dosing for chorioamnionitis. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S68.
Maberry 1991 {published data only}
-
- Maberry M, Gilstrap L, Burris J, Bawdon R, Leveno K. A randomized comparative study of triple antibiotic therapy in the management of acute chorioamnionitis. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:465.
-
- Maberry MC, Gilstrap LC, Bawdon R, Little BB, Dax J. Anaerobic coverage for intra‐amnionic infection: maternal and perinatal impact. American Journal of Perinatology 1991;8:338‐41. - PubMed
Mitra 1997 {published data only}
-
- Mitra AG, Whitten K, Laurent SL, Anderson WE. A randomized, prospective study comparing once‐daily gentamicin versus thrice‐daily gentamicin in the treatment of puerperal infection. American Journal of Obstetrics and Gynecology 1997;177(4):786‐92. - PubMed
Scalambrino 1989 {published data only}
-
- Scalambrino S, Mangioni C, Milani R, Regallo M, Norchi S, Negri L, et al. Sulbactam/ampicillin vs cefotetan in the treatment of obstetric and gynecologic infections. International Journal of Gynecology & Obstetrics 1989;Suppl 2:21‐7. - PubMed
Turnquest 1998 {published data only}
-
- Turnquest MA, How HY, Cook CR, O'Rourke TP, Cureton AC, Spinnato JA, et al. Chorioamnionitis: is continuation of antibiotic therapy necessary after cesarean section?. American Journal of Obstetrics and Gynecology 1998;179(5):1261‐6. - PubMed
References to studies excluded from this review
Budanov 2000 {published data only}
-
- Budanov PV, Baev OR, Musaev ZM. Features of antibacterial therapy intraamniotic infections [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA 2000;Book 1:66.
Creatsas 1980 {published data only}
-
- Creatsas G, Pavlatos M, Lolis D, Kaskarelis D. Ampicillin and gentamicin in the treatment of fetal intrauterine infections. Journal of Perinatal Medicine 1980;8:13‐8. - PubMed
La‐Bella 1996 {published data only}
-
- La‐Bella C, Sandberg P, Edelstone D. Labor‐related intra‐amniotic infection—is postpartum treatment necessary when delivery occurs vaginally?. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):404.
McCredie 1956 {published data only}
-
- McCredie Smith JA, Jennison RF, Langley FA. Perinatal infection and perinatal death: clinical aspects. Lancet 1956;2:903‐6. - PubMed
Rocha 1999 {published data only}
-
- Rocha JE, Duarte G, Cunha S, Nogueira AA, Mauad F. Ampicillin prophylaxis in premature rupture of membranes: randomized and double‐blind study [Antibioticoprofilaxia com ampicilina na rotura prematura das membranas: estudo randomizado e duplo cego]. Revista Brasileira de Ginecologia e Obstetricia 1999;21(5):251‐8.
References to ongoing studies
Aziz 2009 {published data only}
-
- Aziz N. Comparison of ampicillin/sulbactam vs. ampicillin/gentamicin for treatment of intrapartum chorioamnionitis: a randomized controlled trial. ClinicalTrials.gov (https://clinicaltrials.gov/) [accessed 21 May 2013] 2009.
Shanks 2012 {published data only}
-
- Shanks A. Treatment utility of postpartum antibiotics in chorioamnionitis (TUPAC). ClinicalTrials.gov (https://clinicaltrials.gov/) (accessed September 2014). 2012.
Additional references
Baaqeel 2013
Czikk 2011
-
- Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clinical Microbiology and Infection 2011;17(9):1304‐11. - PubMed
Edwards 2005
-
- Edwards R. Chorioamnionitis and labor. Obstetrics and Gynecology Clinics of North America 2005;32:287‐96. - PubMed
Fahey 2008
-
- Fahey J. Clinical management of intra‐amniotic infection and chorioamnionitis: a review of literature. Journal of Midwifery & Women's Health 2008;53(3):227‐35. - PubMed
Fishman 2012
-
- Fishman S, Gelber S. Evidence for the clinical management of chorioamnionitis. Seminars in Fetal and Neonatal Medicine 2012;17:46‐50. - PubMed
Getahun 2010
-
- Getahun D, Strickland D, Zeiger RS, Fassett MJ, Chen W, Rhoads GG, et al. Effect of chorioamnionitis on early childhood asthma. Archives of Pediatrics and Adolescent Medicine 2010;164(2):187‐92. - PubMed
Gibbs 2004
-
- Gibbs R. Management of clinical chorioamnionitis at term. American Journal of Obstetrics and Gynecology 2004;191:1‐2. - PubMed
GRADE 2008 [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Holzman 2007
-
- Holzman C, Lin X, Senagore P, Chung H. Histologic chorioamnionitis and preterm delivery. American Journal of Epidemiology 2007;166(7):786‐94. - PubMed
Incerpi 2010
-
- Incerpi MH. Chorio‐amnionitis and postpartum endometritis. In: Goodwin TM, Montoro MN, Muderspach L, Paulson R, Roy S editor(s). Management of Common Problems in Obstetrics and Gynecology. Oxford: Blackwell Publishing Ltd, 2010.
Kenyon 2013
Lamont 2014
-
- Lamont HF, Blogg HJ, Lamont RF. Safety of antimicrobial treatment during pregnancy: a current review of resistance, immunomodulation and teratogenicity. Expert Opinion on Drug Safety 2014 Sept 5 [Epub ahead of print]:1‐13. [PUBMED: 25189188] - PubMed
López‐Zeno 1992
-
- López‐Zeno JA, Peaceman AM, Adashek JA, Socol ML. A controlled trial of a program for the active management of labor. New England Journal of Medicine 1992;326:450‐4. - PubMed
Manríquez 2008
-
- Manríquez JJ. A highly sensitive search strategy for clinical trials in Literatura Latino Americana e do Caribe em Ciências da Saúde (LILACS) was developed. Journal of Clinical Epidemiology 2008;61(4):407‐11. - PubMed
Mozurkewich 1997
-
- Mozurkeqich E, Wolf F. Premature rupture of membranes at term: a meta‐analyses of three management schemes. Obstetrics and Gynecology 1997;89:1035‐43. - PubMed
Mtira 1997
-
- Mitra A, Whitten K, Laurent S, Anderson W. A randomized, prospective study comparing once‐daily gentamicin versus thrice‐daily gentamicin in the treatment of puerperal infection. American Journal of Obstetrics and Gynecology 1997;177(4):786‐92. - PubMed
Newton 1989
-
- Newton E, Prihoda T, Gibbs R. Logistic regression analysis of risk factors for intra‐amniotic infection. Obstetrics and Gynecology 1989;73(4):571‐5. - PubMed
Ohlsson 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2012
Rocha 2007
-
- Rocha G, Proenca E, Wuintas C, Rodrigues T, Guimaraes H. Chorioanmionitis and brain damage in the preterm newborn. Journal of Maternal‐Fetal and Neonatal Medicine 2007;20(10):745‐9. - PubMed
Schunemann 2009
-
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] - PubMed
Seaward 2005
-
- Seaward G, Hannah M, Myhr T, Farine D, Ohlsson A, Wang E, et al. International multicentre term prelabor rupture of membranes study: evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabor rupture of membranes at term. American Journal of Obstetrics and Gynecology 2005;177(5):1024‐9. - PubMed
Siriwachirachai 2010
Tita 2010
Wu 2000
-
- Wu Y, Colfrod J. Chorioamnionitis as a risk factor for cerebral palsy. JAMA 2000;284(11):1417‐24. - PubMed
Yoon 2001
-
- Yoon B, Romero R, Moon J, Shim S, Kim M, Kim G, et al. Clinical significance of intra‐amniotic inflammation in patients with preterm labor and intact membranes. American Journal of Obstetrics and Gynecology 2001;185(5):1130‐6. - PubMed
References to other published versions of this review
Chapman 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

