From infancy to adolescence: fifteen years of continuous treatment with hydroxyurea in sickle cell anemia
- PMID: 25526439
- PMCID: PMC4603125
- DOI: 10.1097/MD.0000000000000215
From infancy to adolescence: fifteen years of continuous treatment with hydroxyurea in sickle cell anemia
Abstract
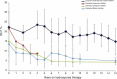
Despite documented laboratory and clinical benefits of hydroxyurea for children with sickle cell anemia (SCA), the drug's long-term safety and efficacy remains poorly defined. The HUSOFT trial and extension study examined feasibility, toxicity, and hematological efficacy of hydroxyurea in infants with SCA. This report describes HUSOFT participants who have continued hydroxyurea therapy for 15 years. With IRB approval, medical records were reviewed for clinical, laboratory, and growth parameters. Twenty-eight infants enrolled in the original 2-year HUSOFT study received open-label liquid hydroxyurea at 20 mg/kg/day; 17 completed the extension study with dose escalation to 30 mg/kg/day. Eight of these 17 (6 girls and 2 boys, all HbSS) have continued on daily hydroxyurea for at least 15 years (median age at last follow-up 17.6 years) without interruption. All hematologic indices (Hb concentration, mean corpuscular volume (MCV), fetal hemoglobin) showed sustained effect after 15 years. The median maximum tolerated dose of hydroxyurea has decreased from 30 to 26 mg/kg/day (range 19.5-31.2); neutropenia [absolute neutrophil count (ANC)<1.0×10⁹/L] prompting temporary drug discontinuation occurred a total of 10 times in 4 subjects and there was no severe neutropenia (ANC<0.5×10⁹/L). Growth rates over 15 years continued at the 50th percentile for both height and weight, and puberty occurred without delay (age range 10-14 years). There were 5.1 vaso-occlusive events (pain and acute chest syndrome)/100 patient years, 7.3 packed red blood cell transfusions/100 patient years. No malignancies, strokes, or deaths occurred. At last follow up, all subjects were at appropriate grade level (10-12 grade) with no history of repeated grades. A cohort of young teenagers with SCA who initiated treatment in infancy have had sustained and continued hematological benefits for a decade and a half. Growth and sexual development are normal and comparable to the general pediatric population. Continuous hydroxyurea therapy since infancy appears safe and efficacious in SCA.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med 1995; 332:1317–1322. - PubMed
-
- Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 2010; 115:2354–2363. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

