Protecting the melatonin rhythm through circadian healthy light exposure
- PMID: 25526564
- PMCID: PMC4284776
- DOI: 10.3390/ijms151223448
Protecting the melatonin rhythm through circadian healthy light exposure
Abstract
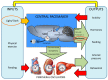
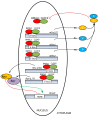
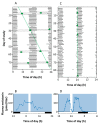
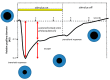
Currently, in developed countries, nights are excessively illuminated (light at night), whereas daytime is mainly spent indoors, and thus people are exposed to much lower light intensities than under natural conditions. In spite of the positive impact of artificial light, we pay a price for the easy access to light during the night: disorganization of our circadian system or chronodisruption (CD), including perturbations in melatonin rhythm. Epidemiological studies show that CD is associated with an increased incidence of diabetes, obesity, heart disease, cognitive and affective impairment, premature aging and some types of cancer. Knowledge of retinal photoreceptors and the discovery of melanopsin in some ganglion cells demonstrate that light intensity, timing and spectrum must be considered to keep the biological clock properly entrained. Importantly, not all wavelengths of light are equally chronodisrupting. Blue light, which is particularly beneficial during the daytime, seems to be more disruptive at night, and induces the strongest melatonin inhibition. Nocturnal blue light exposure is currently increasing, due to the proliferation of energy-efficient lighting (LEDs) and electronic devices. Thus, the development of lighting systems that preserve the melatonin rhythm could reduce the health risks induced by chronodisruption. This review addresses the state of the art regarding the crosstalk between light and the circadian system.
Figures

References
-
- Haim A., Portnov B.A. Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers. Springer Netherlands; Dordrecht, The Netherlands: 2013.
-
- De Beaune S.A., White R. Ice Age Lamps. Sci. Am. 1993;206:108–113. doi: 10.1038/scientificamerican0393-108. - DOI
-
- Nordhaus W.D., Nordhaus W.D. Do real-output and real-wage measures capture reality? The history of lighting suggests not. In: Brenahan T.F., Gordon J., editors. The Economics of New Goods. The University of Chicago Press; Chicago, IL, USA: 1994. pp. 27–70.
-
- Cinzano P., Falchi F., Elvidge C.D. The first world atlas of the artificial night sky brightness. Mon. Not. R. Astron. Soc. 2001;328:689–707. doi: 10.1046/j.1365-8711.2001.04882.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

