Non-microtubular localizations of microtubule-associated protein 6 (MAP6)
- PMID: 25526643
- PMCID: PMC4272302
- DOI: 10.1371/journal.pone.0114905
Non-microtubular localizations of microtubule-associated protein 6 (MAP6)
Abstract
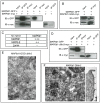
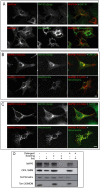
MAP6 proteins (MAP6s), which include MAP6-N (also called Stable Tubule Only Polypeptide, or STOP) and MAP6d1 (MAP6 domain-containing protein 1, also called STOP-Like protein 21 kD, or SL21), bind to and stabilize microtubules. MAP6 deletion in mice severely alters integrated brain functions and is associated with synaptic defects, suggesting that MAP6s may also have alternative cellular roles. MAP6s reportedly associate with the Golgi apparatus through palmitoylation of their N-terminal domain, and specific isoforms have been shown to bind actin. Here, we use heterologous systems to investigate several biochemical properties of MAP6 proteins. We demonstrate that the three N-terminal cysteines of MAP6d1 are palmitoylated by a subset of DHHC-type palmitoylating enzymes. Analysis of the subcellular localization of palmitoylated MAP6d1, including electron microscopic analysis, reveals possible localization to the Golgi and the plasma membrane but no association with the endoplasmic reticulum. Moreover, we observed localization of MAP6d1 to mitochondria, which requires the N-terminus of the protein but does not require palmitoylation. We show that endogenous MAP6d1 localized at mitochondria in mature mice neurons as well as at the outer membrane and in the intermembrane space of purified mouse mitochondria. Last, we found that MAP6d1 can multimerize via a microtubule-binding module. Interestingly, most of these properties of MAP6d1 are shared by MAP6-N. Together, these results describe several properties of MAP6 proteins, including their intercellular localization and multimerization activity, which may be relevant to neuronal differentiation and synaptic functions.
Conflict of interest statement
Figures




References
-
- Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13:83–117. - PubMed
-
- Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242. - PubMed
-
- Job D, Margolis RL (1984) Isolation from bovine brain of a superstable microtubule subpopulation with microtubule seeding activity. Biochemistry 23:3025–3031. - PubMed
-
- Baas PW, Slaughter T, Brown A, Black MM (1991) Microtubule dynamics in axons and dendrites. J Neurosci Res 30:134–153. - PubMed
-
- Gory-Faure S, Windscheid V, Bosc C, Peris L, Proietto D, et al. (2006) STOP-like protein 21 is a novel member of the STOP family, revealing a Golgi localization of STOP proteins. J Biol Chem 281:28387–28396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

