Randomized Trial of Periodic Presumptive Treatment With High-Dose Intravaginal Metronidazole and Miconazole to Prevent Vaginal Infections in HIV-negative Women
- PMID: 25526757
- PMCID: PMC4836721
- DOI: 10.1093/infdis/jiu818
Randomized Trial of Periodic Presumptive Treatment With High-Dose Intravaginal Metronidazole and Miconazole to Prevent Vaginal Infections in HIV-negative Women
Abstract
Background: Vaginal infections are common, frequently recur, and may increase women's risk for sexually transmitted infections (STIs). We tested the efficacy of a novel regimen to prevent recurrent vaginal infections.
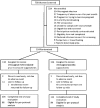
Methods: Human immunodeficiency virus (HIV)-negative women 18-45 years old with 1 or more vaginal infections, including bacterial vaginosis (BV), vulvovaginal candidiasis (VVC), or Trichomonas vaginalis (TV), were randomly assigned to receive vaginal suppositories containing metronidazole 750 mg plus miconazole 200 mg or matching placebo for 5 consecutive nights each month for 12 months. Primary endpoints, evaluated every 2 months, were BV (Gram stain) and VVC (positive wet mount and culture).
Results: Participants (N = 234) were randomly assigned to the intervention (N = 118) or placebo (N = 116) arm. Two hundred seventeen (93%) women completed an end-of-study evaluation. The intervention reduced the proportion of visits with BV compared to placebo (21.2% vs 32.5%; relative risk [RR] 0.65, 95% confidence interval [CI] .48-.87). In contrast, the proportion of visits with VVC was similar in the intervention (10.4%) versus placebo (11.3%) arms (RR 0.92, 95% CI .62-1.37).
Conclusions: Monthly treatment with intravaginal metronidazole plus miconazole reduced the proportion of visits with BV during 12 months of follow-up. Further study will be important to determine whether this intervention can reduce women's risk of STIs.
Keywords: Trichomonas vaginalis; bacterial vaginosis; metronidazole; miconazole; periodic presumptive treatment; vulvovaginal candidiasis.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Martin HL, Jr, Nyange PM, Richarson BA, et al. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis 1998; 178:1053–59. - PubMed
-
- Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 12:1699–706. - PubMed
-
- Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis 2005; 192:1372–80. - PubMed
-
- van de Wijgert JH, Morrison CS, Cornelisse PG, et al. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 2008; 48:203–10. - PubMed
-
- Kilmarx PH, Limpakarnjanarat K, Mastro TD, et al. HIV-1 seroconverson in a prospective study of female sex workers in northern Thailand: continued high incidence among brothel-based women. AIDS 1998; 12:1889–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

