Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS
- PMID: 25526880
- PMCID: PMC4272556
- DOI: 10.1186/s12968-014-0102-0
Simultaneous three-dimensional myocardial T1 and T2 mapping in one breath hold with 3D-QALAS
Abstract
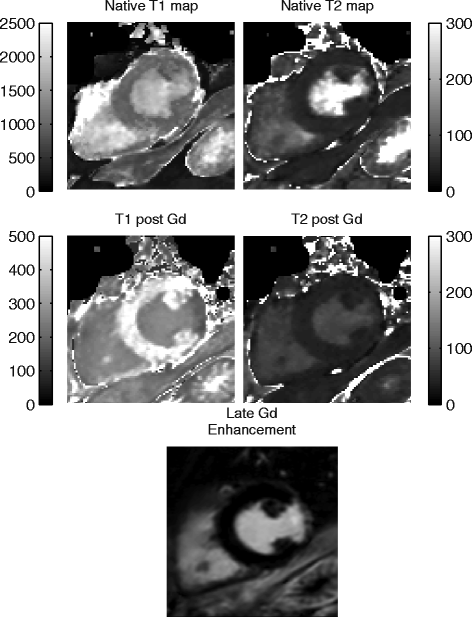
Background: Quantification of the longitudinal- and transverse relaxation time in the myocardium has shown to provide important information in cardiac diagnostics. Methods for cardiac relaxation time mapping generally demand a long breath hold to measure either T1 or T2 in a single 2D slice. In this paper we present and evaluate a novel method for 3D interleaved T1 and T2 mapping of the whole left ventricular myocardium within a single breath hold of 15 heartbeats.
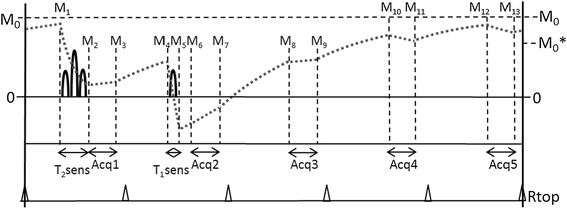
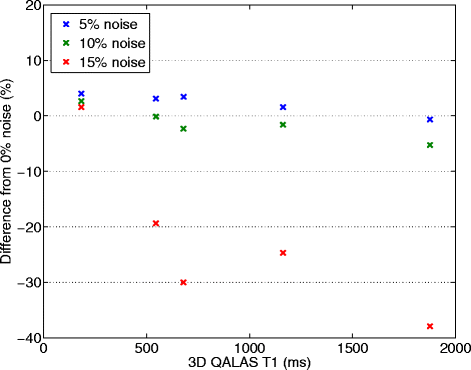
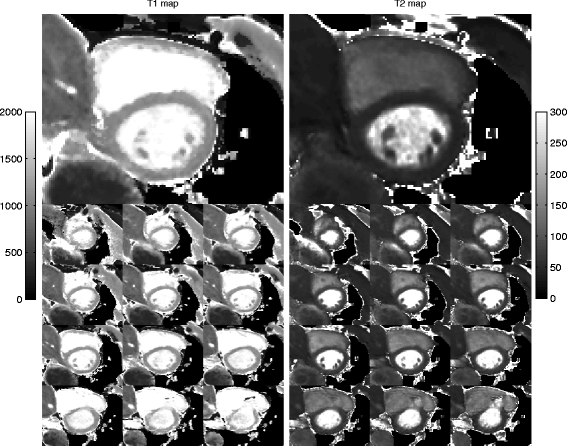
Methods: The 3D-QALAS (3D-quantification using an interleaved Look-Locker acquisition sequence with T2 preparation pulse) is based on a 3D spoiled Turbo Field Echo sequence using inversion recovery with interleaved T2 preparation. Quantification of both T1 and T2 in a volume of 13 slices with a resolution of 2.0x2.0x6.0 mm is obtained from five measurements by using simulations of the longitudinal magnetizations Mz. This acquisition scheme is repeated three times to sample k-space. The method was evaluated both in-vitro (validated against Inversion Recovery and Multi Echo) and in-vivo (validated against MOLLI and Dual Echo).
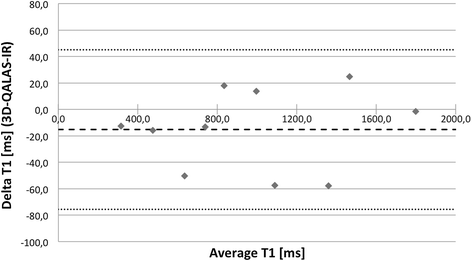
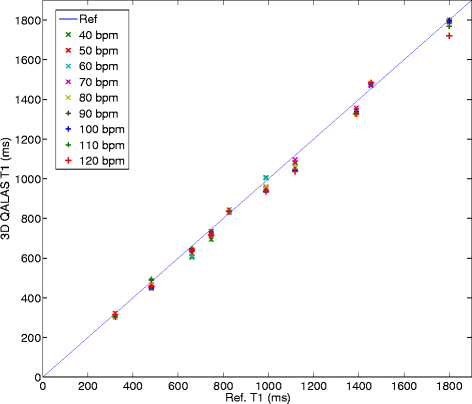
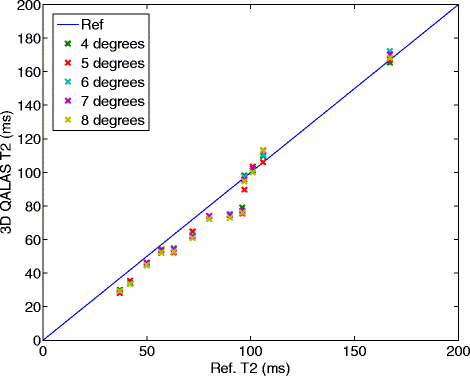
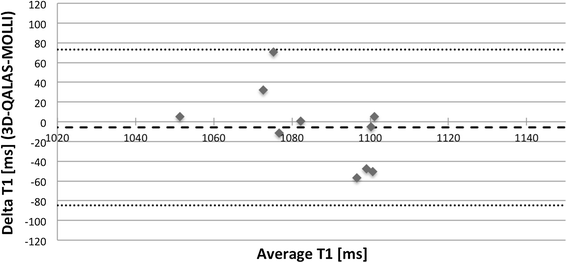
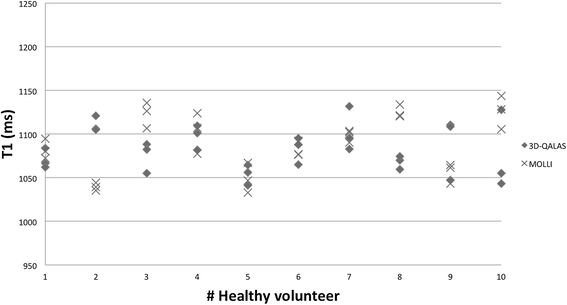
Results: In-vitro, a strong relation was found between 3D-QALAS and Inversion Recovery (R = 0.998; N = 10; p < 0.01) and between 3D-QALAS and Multi Echo (R = 0.996; N = 10; p < 0.01). The 3D-QALAS method showed no dependence on e.g. heart rate in the interval of 40-120 bpm. In healthy myocardium, the mean T1 value was 1083 ± 43 ms (mean ± SD) for 3D-QALAS and 1089 ± 54 ms for MOLLI, while the mean T2 value was 50.4 ± 3.6 ms 3D-QALAS and 50.3 ± 3.5 ms for Dual Echo. No significant difference in in-vivo relaxation times was found between 3D-QALAS and MOLLI (N = 10; p = 0.65) respectively 3D-QALAS and Dual Echo (N = 10; p = 0.925) for the ten healthy volunteers.
Conclusions: The 3D-QALAS method has demonstrated good accuracy and intra-scan variability both in-vitro and in-vivo. It allows rapid acquisition and provides quantitative information of both T1 and T2 relaxation times in the same scan with full coverage of the left ventricle, enabling clinical application in a broader spectrum of cardiac disorders.
Figures






References
-
- Chang KJ, Jara H. Applications of quantitative T1, T2, and proton density to diagnosis.Appl Radiol. 2005; 34–42.
-
- Riederer S, Lee J, Farzaneh F, Wang H, Wright R. Magnetic resonance image synthesis. clinical implementation. Acta Radiol Suppl. 1986;369:466. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

