In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography
- PMID: 25526974
- PMCID: PMC4339768
- DOI: 10.1093/brain/awu344
In vivo detection of nerve injury in familial amyloid polyneuropathy by magnetic resonance neurography
Abstract
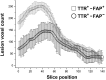
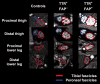
Transthyretin familial amyloid polyneuropathy is a rare, autosomal-dominant inherited multisystem disorder usually manifesting with a rapidly progressive, axonal, distally-symmetric polyneuropathy. The detection of nerve injury by nerve conduction studies is limited, due to preferential involvement of small-fibres in early stages. We investigated whether lower limb nerve-injury can be detected, localized and quantified in vivo by high-resolution magnetic resonance neurography. We prospectively included 20 patients (12 male and eight female patients, mean age 47.9 years, range 26-66) with confirmed mutation in the transthyretin gene: 13 with symptomatic polyneuropathy and seven asymptomatic gene carriers. A large age- and sex-matched cohort of healthy volunteers served as controls (20 male and 20 female, mean age 48.1 years, range 30-73). All patients received detailed neurological and electrophysiological examinations and were scored using the Neuropathy Impairment Score-Lower Limbs, Neuropathy Deficit and Neuropathy Symptom Score. Magnetic resonance neurography (3 T) was performed with large longitudinal coverage from proximal thigh to ankle-level and separately for each leg (140 axial slices/leg) by using axial T2-weighted (repetition time/echo time = 5970/55 ms) and dual echo (repetition time 5210 ms, echo times 12 and 73 ms) turbo spin echo 2D sequences with spectral fat saturation. A 3D T2-weighted inversion-recovery sequence (repetition time/echo time 3000/202 ms) was acquired for imaging of the spinal nerves and lumbar plexus (50 axial slice reformations). Precise manual segmentation of the spinal/sciatic/tibial/common peroneal nerves was performed on each slice. Histogram-based normalization of nerve-voxel signal intensities was performed using the age- and sex-matched control group as normative reference. Nerve-voxels were subsequently classified as lesion-voxels if a threshold of >1.2 (normalized signal-intensity) was exceeded. At distal thigh level, where a predominant nerve-lesion-voxel burden was observed, signal quantification was performed by calculating proton spin density and T2-relaxation time as microstructural markers of nerve tissue integrity. The total number of nerve-lesion voxels (cumulated from proximal-to-distal) was significantly higher in symptomatic patients (20 405 ± 1586) versus asymptomatic gene carriers (12 294 ± 3199; P = 0.036) and versus controls (6536 ± 467; P < 0.0001). It was also higher in asymptomatic carriers compared to controls (P = 0.043). The number of nerve-lesion voxels was significantly higher at thigh level compared to more distal levels (lower leg/ankle) of the lower extremities (f-value = 279.22, P < 0.0001). Further signal-quantification at this proximal site (thigh level) revealed a significant increase of proton-density (P < 0.0001) and T2-relaxation-time (P = 0.0011) in symptomatic patients, whereas asymptomatic gene-carriers presented with a significant increase of proton-density only. Lower limb nerve injury could be detected and quantified in vivo on microstructural level by magnetic resonance neurography in symptomatic familial amyloid polyneuropathy, and also in yet asymptomatic gene carriers, in whom imaging detection precedes clinical and electrophysiological manifestation. Although symptoms start and prevail distally, the focus of predominant nerve injury and injury progression was found proximally at thigh level with strong and unambiguous lesion-contrast. Imaging of proximal nerve lesions, which are difficult to detect by nerve conduction studies, may have future implications also for other distally-symmetric polyneuropathies.
Keywords: MR imaging; MR neurography; amyloid polyneuropathy; hereditary amyloidosis; transthyretin familial amyloid polyneuropathy.
© The Author (2014). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



Comment in
-
Early detection of nerve injury in transthyretin-related familial amyloid polyneuropathy.Brain. 2015 Mar;138(Pt 3):507-9. doi: 10.1093/brain/awu396. Brain. 2015. PMID: 25713400 Free PMC article.
References
-
- Abbas Z, Gras V, Mollenhoff K, Keil F, Oros-Peusquens AM, Shah NJ. Analysis of proton-density bias corrections based on T measurement for robust quantification of water content in the brain at 3 Tesla. Magn Reson Med. 2014;72:1735–45. - PubMed
-
- Adams D, Samuel D, Goulon-Goeau C, Nakazato M, Costa PM, Feray C, et al. The course and prognostic factors of familial amyloid polyneuropathy after liver transplantation. Brain. 2000;123 (Pt 7):1495–504. - PubMed
-
- Andrade C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–27. - PubMed
-
- Bendszus M, Stoll G. Technology insight: visualizing peripheral nerve injury using MRI. Nat Clin Pract Neurol. 2005;1:45–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

