Expression profiling and structural characterization of microRNAs in adipose tissues of hibernating ground squirrels
- PMID: 25526980
- PMCID: PMC4411486
- DOI: 10.1016/j.gpb.2014.08.003
Expression profiling and structural characterization of microRNAs in adipose tissues of hibernating ground squirrels
Abstract
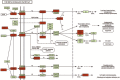
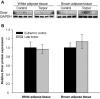
MicroRNAs (miRNAs) are small non-coding RNAs that are important in regulating metabolic stress. In this study, we determined the expression and structural characteristics of 20 miRNAs in brown (BAT) and white adipose tissue (WAT) during torpor in thirteen-lined ground squirrels. Using a modified stem-loop technique, we found that during torpor, expression of six miRNAs including let-7a, let-7b, miR-107, miR-150, miR-222 and miR-31 was significantly downregulated in WAT (P<0.05), which was 16%-54% of euthermic non-torpid control squirrels, whereas expression of three miRNAs including miR-143, miR-200a and miR-519d was found to be upregulated by 1.32-2.34-fold. Similarly, expression of more miRNAs was downregulated in BAT during torpor. We detected reduced expression of 6 miRNAs including miR-103a, miR-107, miR-125b, miR-21, miR-221 and miR-31 (48%-70% of control), while only expression of miR-138 was significantly upregulated (2.91±0.8-fold of the control, P<0.05). Interestingly, miRNAs found to be downregulated in WAT during torpor were similar to those dysregulated in obese humans for increased adipogenesis, whereas miRNAs with altered expression in BAT during torpor were linked to mitochondrial β-oxidation. miRPath target prediction analysis showed that miRNAs downregulated in both WAT and BAT were associated with the regulation of mitogen-activated protein kinase (MAPK) signaling, while the miRNAs upregulated in WAT were linked to transforming growth factor β (TGFβ) signaling. Compared to mouse sequences, no unique nucleotide substitutions within the stem-loop region were discovered for the associated pre-miRNAs for the miRNAs used in this study, suggesting no structure-influenced changes in pre-miRNA processing efficiency in the squirrel. As well, the expression of miRNA processing enzyme Dicer remained unchanged in both tissues during torpor. Overall, our findings suggest that changes of miRNA expression in adipose tissues may be linked to distinct biological roles in WAT and BAT during hibernation and may involve the regulation of signaling cascades.
Keywords: Dicer; Ground squirrel; Hypometabolism; Non-coding RNA; Stress adaptation.
Copyright © 2014 The Authors. Production and hosting by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Wang L.C.H., Wolowyk M. Torpor in mammals and birds. Can J Zool. 1987;66:133–137.
-
- McArthur M.D., Milsom W.K. Changes in ventilation and respiratory sensitivity associated with hibernation in Columbian (Spermophilus columbianus) and golden-mantled (Spermophilus lateralis) ground squirrels. Physiol Zool. 1991;64:940–959.
-
- Heldmaier G., Ortmann S., Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. - PubMed
-
- Sheriff M.J., Fridinger R.W., Tøien Ø., Barnes B.M., Buck C.L. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool. 2013;86:515–527. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

