Colonization in the photic zone and subsequent changes during sinking determine bacterial community composition in marine snow
- PMID: 25527538
- PMCID: PMC4309695
- DOI: 10.1128/AEM.02570-14
Colonization in the photic zone and subsequent changes during sinking determine bacterial community composition in marine snow
Abstract
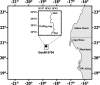
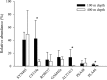
Due to sampling difficulties, little is known about microbial communities associated with sinking marine snow in the twilight zone. A drifting sediment trap was equipped with a viscous cryogel and deployed to collect intact marine snow from depths of 100 and 400 m off Cape Blanc (Mauritania). Marine snow aggregates were fixed and washed in situ to prevent changes in microbial community composition and to enable subsequent analysis using catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH). The attached microbial communities collected at 100 m were similar to the free-living community at the depth of the fluorescence maximum (20 m) but different from those at other depths (150, 400, 550, and 700 m). Therefore, the attached microbial community seemed to be “inherited” from that at the fluorescence maximum. The attached microbial community structure at 400 m differed from that of the attached community at 100 m and from that of any free-living community at the tested depths, except that collected near the sediment at 700 m. The differences between the particle-associated communities at 400 m and 100 m appeared to be due to internal changes in the attached microbial community rather than de novo colonization, detachment, or grazing during the sinking of marine snow. The new sampling method presented here will facilitate future investigations into the mechanisms that shape the bacterial community within sinking marine snow, leading to better understanding of the mechanisms which regulate biogeochemical cycling of settling organic matter.
Figures


References
-
- Volk T, Hoffert MI. 1985. Ocean carbon pumps: analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes, p 99-110. In Sundquist ET, Broecker WS (ed), The carbon cycle and atmospheric CO2: natural variations archean to present, vol 32 AGU, Washington, DC.
-
- Siegenthaler U, Sarmiento JL. 1993. Atmospheric carbon dioxide and the ocean. Nature 365:119–125. doi:10.1038/365119a0. - DOI
-
- Urban-Rich J. 1999. Release of dissolved organic carbon from copepod fecal pellets in the Greenland Sea. J Exp Mar Biol Ecol 232:107–124. doi:10.1016/S0022-0981(98)00104-X. - DOI
-
- Simon M, Grossart HP, Schweitzer B, Ploug H. 2002. Microbial ecology of organic aggregates in aquatic ecosystems. Aquat Microb Ecol 28:175–211. doi:10.3354/ame028175. - DOI
-
- DeLong EF, Franks DG, Alldredge A. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacteria assemblages. Limnol Oceanogr 38:924–934. doi:10.4319/lo.1993.38.5.0924. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

