Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial
- PMID: 25528956
- PMCID: PMC4361328
- DOI: 10.1016/j.ajo.2014.12.013
Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial
Abstract
Purpose: To evaluate the safety and tolerability of intraocular delivery of ciliary neurotrophic factor (CNTF) using an encapsulated cell implant for the treatment of macular telangiectasia type 2.
Design: An open-label safety trial conducted in 2 centers enrolling 7 participants with macular telangiectasia type 2.
Methods: The participant's more severely affected eye (worse baseline visual acuity) received the high-dose implant of CNTF. Patients were followed for a period of 36 months. The primary safety outcome was a change in the parameters of the electroretinogram (ERG). Secondary efficacy outcomes were changes in visual acuity, en face measurements of the optical coherence tomography of the disruption in the ellipsoid zone, and microperimetry when compared with baseline.
Results: The ERG findings demonstrated a reduction in the amplitude of the scotopic b-wave in 4 participants 3 months after implantation (month 3). All parameters returned to baseline values by month 12 and remained so at month 36 with no clinical impact on dark adaptation. There was no change in visual acuity compared with baseline. The area of the defect as measured functionally by microperimetry and structurally by the en face OCT imaging of the ellipsoid zone loss appeared unchanged from baseline.
Conclusions: The intraocular delivery of CNTF in the encapsulated cell implant appeared to be safe and well tolerated in eyes with macular telangiectasia type 2. Further evaluation in a randomized controlled clinical trial is warranted to test for efficacy.
Published by Elsevier Inc.
Figures
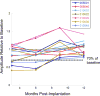


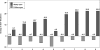
References
-
- Finger RP, Charbel Issa P, Fimmers R, Holz FG, Rubin GS, Scholl HP. Reading performance is reduced by parafoveal scotomas in patients with macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2009;50(3):1366–1370. - PubMed
-
- Maruko I, Iida T, Sekiryu T, Fujiwara T. Early morphological changes and functional abnormalities in group 2A idiopathic juxtafoveolar retinal telangiectasis using spectral domain optical coherence tomography and microperimetry. Br J Ophthalmol. 2008;92(11):1488–1491. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

