Synthetic approaches to the lamellarins--a comprehensive review
- PMID: 25528958
- PMCID: PMC4278223
- DOI: 10.3390/md12126142
Synthetic approaches to the lamellarins--a comprehensive review
Abstract
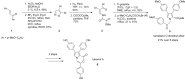
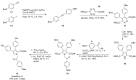
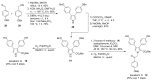
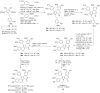
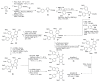
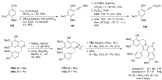
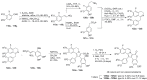
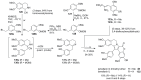
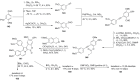
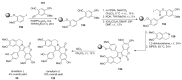
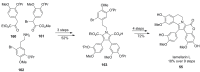
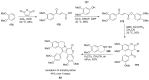
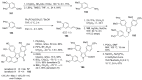
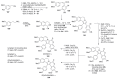
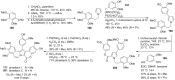
The present review discusses the known synthetic routes to the lamellarin alkaloids published until 2014. It begins with syntheses of the structurally simpler type-II lamellarins and then focuses on the larger class of the 5,6-saturated and -unsaturated type-I lamellarins. The syntheses are grouped by the strategy employed for the assembly of the central pyrrole ring.
Figures




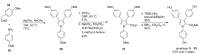












References
-
- Andersen R.J., Faulkner D.J., He C.H., Van Duyne G.D., Clardy J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985;107:5492–5495. doi: 10.1021/ja00305a027. - DOI
-
- Lindquist N., Fenical W., van Duyne G.D., Clardy J. New alkaloids of the lamellarin class from the marine ascidian Didemnum chartaceum. J. Org. Chem. 1988;53:4570–4574. doi: 10.1021/jo00254a029. - DOI
-
- Carroll A.R., Bowden B.F., Coll J.C. Studies of Australian ascidians. I. Six new lamellarin-class alkaloids from a colonial ascidian, Didemnum sp. Aust. J. Chem. 1993;46:489–501. doi: 10.1071/CH9930489. - DOI
-
- Urban S., Butler M.S., Capon R.J. Lamellarins O and P: New aromatic metabolites from the Australian marine sponge Dendrilla cactos. Aust. J. Chem. 1994;47:1919–1924. doi: 10.1071/CH9941919. - DOI
-
- Urban S., Hobbs L., Hooper J.N.A., Capon R.J. Lamellarins Q and R: New aromatic metabolites from an Australian marine sponge, Dendrilla cactos. Aust. J. Chem. 1995;48:1491–1494. doi: 10.1071/CH9951491. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

