CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice
- PMID: 25529917
- PMCID: PMC4406806
- DOI: 10.1002/hep.27665
CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice
Abstract
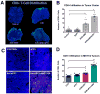
Sorafenib, a broad tyrosine kinase inhibitor, is the only approved systemic therapy for advanced hepatocellular carcinoma (HCC) but provides limited survival benefits. Recently, immunotherapy has emerged as a promising treatment strategy, but its role remains unclear in HCCs, which are associated with decreased cytotoxic CD8(+) T-lymphocyte infiltration in both murine and human tumors. Moreover, in mouse models after sorafenib treatment intratumoral hypoxia is increased and may fuel evasive resistance. Using orthotopic HCC models, we now show that increased hypoxia after sorafenib treatment promotes immunosuppression, characterized by increased intratumoral expression of the immune checkpoint inhibitor programmed death ligand-1 and accumulation of T-regulatory cells and M2-type macrophages. We also show that the recruitment of immunosuppressive cells is mediated in part by hypoxia-induced up-regulation of stromal cell-derived 1 alpha. Inhibition of the stromal cell-derived 1 alpha receptor (C-X-C receptor type 4 or CXCR4) using AMD3100 prevented the polarization toward an immunosuppressive microenvironment after sorafenib treatment, inhibited tumor growth, reduced lung metastasis, and improved survival. However, the combination of AMD3100 and sorafenib did not significantly change cytotoxic CD8(+) T-lymphocyte infiltration into HCC tumors and did not modify their activation status. In separate experiments, antibody blockade of the programmed death ligand-1 receptor programmed death receptor-1 (PD-1) showed antitumor effects in treatment-naive tumors in orthotopic (grafted and genetically engineered) models of HCC. However, anti-PD-1 antibody treatment had additional antitumor activity only when combined with sorafenib and AMD3100 and not when combined with sorafenib alone.
Conclusion: Anti-PD-1 treatment can boost antitumor immune responses in HCC models; when used in combination with sorafenib, anti-PD-1 immunotherapy shows efficacy only with concomitant targeting of the hypoxic and immunosuppressive microenvironment with agents such as CXCR4 inhibitors.
© 2014 by the American Association for the Study of Liver Diseases.
Figures






References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
