The therapeutic monoclonal antibody market
- PMID: 25529996
- PMCID: PMC4622599
- DOI: 10.4161/19420862.2015.989042
The therapeutic monoclonal antibody market
Abstract
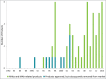
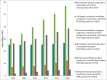
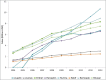
Since the commercialization of the first therapeutic monoclonal antibody product in 1986, this class of biopharmaceutical products has grown significantly so that, as of November 10, 2014, forty-seven monoclonal antibody products have been approved in the US or Europe for the treatment of a variety of diseases, and many of these products have also been approved for other global markets. At the current approval rate of ∼ four new products per year, ∼ 70 monoclonal antibody products will be on the market by 2020, and combined world-wide sales will be nearly $125 billion.
Figures
References
-
- Bioprocess Technology Consultants [Internet] Woburn (MA): Bioprocess Technology Consultants, Inc. bioTRAK® database; 2014. [cited 2014 September 14]; [about 2 screens] Available from: http://www.bptc.com/pipeline-databases.php
-
- Pharmaceutical Research and Manufacturers of America Medicines in development: Biologics 2013 Report [Internet]. Washington, DC: Pharmaceutical Research and Manufacturers of America; 2013 Mar. [cited 2014 September 14] 87 p. Available from: http://www.phrma.org/sites/default/files/pdf/biologics2013.pdf
-
- Drugs@FDA [Internet] Silver Spring (MD): U.S. Food and Drug Administration, Center for Drug Evaluation and Research. 1939- [cited 2014 September 14] Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm
-
- European public assessment reports, human medicines [Internet] London: The European Medicines Agency. 1995. [cited 2014 September 14] Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar...
-
- Ecker DM, Ransohoff TC, Jones SD, Levine HL. The state of mammalian cell culture biomanufacturing. Woburn: (MA: ): BioProcess Technology Consultants, Inc; 2011 Dec 12; 150 p. Available from: http://www.bptc.com/ECommerce/Reports.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
