Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells
- PMID: 25530098
- PMCID: PMC4324584
- DOI: 10.3892/ijo.2014.2804
Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells
Abstract
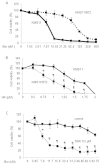
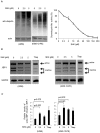
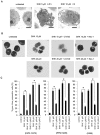
Shikonin (SHK), a natural small agent (MW 288.3), reportedly induces cell death in various tumor cells. We have found that SHK also exerts potent cytocidal effects on human multiple myeloma (MM) cells, but its anticancer mechanism in MM cells remains to be elucidated. SHK at 2.5-5 µM induced apoptosis in seven MM cell lines, including the bortezomib-resistant cell line KMS11/BTZ. The IC50 value of SHK against KMS11/BTZ was comparable to that of a parental cell line KMS11 (1.1 and 1.56 µM, respectively). SHK induces accumulation of ubiquitinated proteins and activates XBP-1 in MM cells, suggesting that SHK functions as a proteasome inhibitor, eventually inducing ER stress-associated apoptosis. SHK increases levels of HSP70/72, which protects cells from apoptosis, and exerts greater cytocidal effects in combination with the HSP70/72 inhibitor VER-155008. At higher concentrations (10-20 µM), SHK induced cell death, which was completely inhibited by a necroptosis inhibitor, necrostatin-1 (Nec-1), while the cytocidal activity was unaffected by Z-VAD-FMK, strongly suggesting that cell death is induced by SHK at high concentrations through necroptosis. The present data show for the first time that SHK induces cell death in MM cells. SHK efficiently induces apoptosis and combination of heat shock protein inhibitor with low dose SHK enhances apoptosis, while high dose SHK induces necroptosis in MM cells. These findings together support the use of SHK as a potential therapeutic agent for MM.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

