Nano-gold corking and enzymatic uncorking of carbon nanotube cups
- PMID: 25530234
- PMCID: PMC4308760
- DOI: 10.1021/ja511843w
Nano-gold corking and enzymatic uncorking of carbon nanotube cups
Abstract
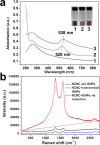
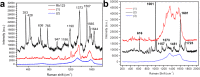
Because of their unique stacked, cup-shaped, hollow compartments, nitrogen-doped carbon nanotube cups (NCNCs) have promising potential as nanoscale containers. Individual NCNCs are isolated from their stacked structure through acid oxidation and subsequent probe-tip sonication. The NCNCs are then effectively corked with gold nanoparticles (GNPs) by sodium citrate reduction with chloroauric acid, forming graphitic nanocapsules with significant surface-enhanced Raman signature. Mechanistically, the growth of the GNP corks starts from the nucleation and welding of gold seeds on the open rims of NCNCs enriched with nitrogen functionalities, as confirmed by density functional theory calculations. A potent oxidizing enzyme of neutrophils, myeloperoxidase (MPO), can effectively open the corked NCNCs through GNP detachment, with subsequent complete enzymatic degradation of the graphitic shells. This controlled opening and degradation was further carried out in vitro with human neutrophils. Furthermore, the GNP-corked NCNCs were demonstrated to function as novel drug delivery carriers, capable of effective (i) delivery of paclitaxel to tumor-associated myeloid-derived suppressor cells (MDSC), (ii) MPO-regulated release, and (iii) blockade of MDSC immunosuppressive potential.
Figures





References
-
- Matsumura Y.; Maeda H. Cancer Res. 1986, 46, 6387. - PubMed
-
- Allen T. M.; Cullis P. R. Science 2004, 303, 1818. - PubMed
-
- O’Neal D. P.; Hirsch L. R.; Halas N. J.; Payne J. D.; West J. L. Cancer Lett. 2004, 209, 171. - PubMed
-
- Maeda H. J. Controlled Release 2012, 164, 138. - PubMed
-
- Portney N. G.; Ozkan M. Anal. Bioanal. Chem. 2006, 384, 620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

