Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss
- PMID: 25530343
- PMCID: PMC4722865
- DOI: 10.1002/hipo.22405
Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss
Abstract
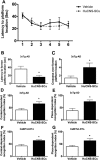
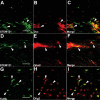
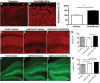
Alzheimer's disease (AD) is the most prevalent age-related neurodegenerative disorder, affecting over 35 million people worldwide. Pathologically, AD is characterized by the progressive accumulation of β-amyloid (Aβ) plaques and neurofibrillary tangles within the brain. Together, these pathologies lead to marked neuronal and synaptic loss and corresponding impairments in cognition. Current treatments, and recent clinical trials, have failed to modify the clinical course of AD; thus, the development of novel and innovative therapies is urgently needed. Over the last decade, the potential use of stem cells to treat cognitive impairment has received growing attention. Specifically, neural stem cell transplantation as a treatment for AD offers a novel approach with tremendous therapeutic potential. We previously reported that intrahippocampal transplantation of murine neural stem cells (mNSCs) can enhance synaptogenesis and improve cognition in 3xTg-AD mice and the CaM/Tet-DT(A) model of hippocampal neuronal loss. These promising findings prompted us to examine a human neural stem cell population, HuCNS-SC, which has already been clinically tested for other neurodegenerative disorders. In this study, we provide the first evidence that transplantation of research grade HuCNS-SCs can improve cognition in two complementary models of neurodegeneration. We also demonstrate that HuCNS-SC cells can migrate and differentiate into immature neurons and glia and significantly increase synaptic and growth-associated markers in both 3xTg-AD and CaM/Tet-DTA mice. Interestingly, improvements in aged 3xTg-AD mice were not associated with altered Aβ or tau pathology. Rather, our findings suggest that human NSC transplantation improves cognition by enhancing endogenous synaptogenesis. Taken together, our data provide the first preclinical evidence that human NSC transplantation could be a safe and effective therapeutic approach for treating AD.
Keywords: Alzheimer's disease; immune suppression; neural stem cells; therapeutic; transplantation.
© 2014 The Authors. Hippocampus Published by Wiley Periodicals, Inc.
Figures



References
-
- Baglietto‐Vargas D, Moreno‐Gonzalez I, Sanchez‐Varo R, Jimenez S, Trujillo‐Estrada L, Sanchez‐Mejias E, Torres M, Romero‐Acebal M, Ruano D, Vizuete M, J Vitorica, A Gutierrez. 2010. Calretinin interneurons are early targets of extracellular amyloid‐beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. J Alzheimer's Dis 21:119–132. - PubMed
-
- Basi GS, Jacobson RD, Virag I, Schilling J, Skene JH. 1987. Primary structure and transcriptional regulation of GAP‐43, a protein associated with nerve growth. Cell 49:785–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

