LARP1 post-transcriptionally regulates mTOR and contributes to cancer progression
- PMID: 25531318
- PMCID: PMC4430325
- DOI: 10.1038/onc.2014.428
LARP1 post-transcriptionally regulates mTOR and contributes to cancer progression
Abstract
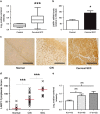
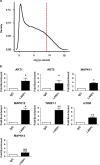
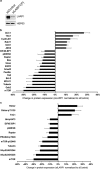
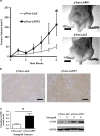
RNA-binding proteins (RBPs) bind to and post-transcriptionally regulate the stability of mRNAs. La-related protein 1 (LARP1) is a conserved RBP that interacts with poly-A-binding protein and is known to regulate 5'-terminal oligopyrimidine tract (TOP) mRNA translation. Here, we show that LARP1 is complexed to 3000 mRNAs enriched for cancer pathways. A prominent member of the LARP1 interactome is mTOR whose mRNA transcript is stabilized by LARP1. At a functional level, we show that LARP1 promotes cell migration, invasion, anchorage-independent growth and in vivo tumorigenesis. Furthermore, we show that LARP1 expression is elevated in epithelial cancers such as cervical and non-small cell lung cancers, where its expression correlates with disease progression and adverse prognosis, respectively. We therefore conclude that, through the post-transcriptional regulation of genes such as mTOR within cancer pathways, LARP1 contributes to cancer progression.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

