PARP inhibition restores extrinsic apoptotic sensitivity in glioblastoma
- PMID: 25531448
- PMCID: PMC4273972
- DOI: 10.1371/journal.pone.0114583
PARP inhibition restores extrinsic apoptotic sensitivity in glioblastoma
Abstract
Background: Resistance to apoptosis is a paramount issue in the treatment of Glioblastoma (GBM). We show that targeting PARP by the small molecule inhibitors, Olaparib (AZD-2281) or PJ34, reduces proliferation and lowers the apoptotic threshold of GBM cells in vitro and in vivo.
Methods: The sensitizing effects of PARP inhibition on TRAIL-mediated apoptosis and potential toxicity were analyzed using viability assays and flow cytometry in established GBM cell lines, low-passage neurospheres and astrocytes in vitro. Molecular analyses included western blots and gene silencing. In vivo, effects on tumor growth were examined in a murine subcutaneous xenograft model.
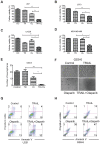
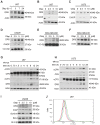
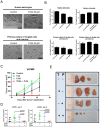
Results: The combination treatment of PARP inhibitors and TRAIL led to an increased cell death with activation of caspases and inhibition of formation of neurospheres when compared to single-agent treatment. Mechanistically, pharmacological PARP inhibition elicited a nuclear stress response with up-regulation of down-stream DNA-stress response proteins, e.g., CCAAT enhancer binding protein (C/EBP) homology protein (CHOP). Furthermore, Olaparib and PJ34 increased protein levels of DR5 in a concentration and time-dependent manner. In turn, siRNA-mediated suppression of DR5 mitigated the effects of TRAIL/PARP inhibitor-mediated apoptosis. In addition, suppression of PARP-1 levels enhanced TRAIL-mediated apoptosis in malignant glioma cells. Treatment of human astrocytes with the combination of TRAIL/PARP inhibitors did not cause toxicity. Finally, the combination treatment of TRAIL and PJ34 significantly reduced tumor growth in vivo when compared to treatment with each agent alone.
Conclusions: PARP inhibition represents a promising avenue to overcome apoptotic resistance in GBM.
Conflict of interest statement
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. - PubMed
-
- von Minckwitz G, Muller BM, Loibl S, Budczies J, Hanusch C, et al. (2011) Cytoplasmic poly(adenosine diphosphate-ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 29:2150–2157. - PubMed
-
- Gan A, Green AR, Nolan CC, Martin S, Deen S (2013) Poly(adenosine diphosphate-ribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum Pathol 44:1638–1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

