Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice
- PMID: 25531553
- PMCID: PMC4615720
- DOI: 10.4161/19490976.2014.990790
Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice
Abstract
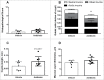
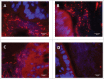
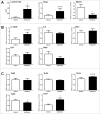
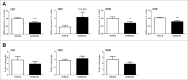
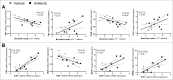
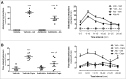
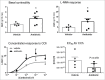
Alterations in the composition of the commensal microbiota (dysbiosis) seem to be a pathogenic component of functional gastrointestinal disorders, mainly irritable bowel syndrome (IBS), and might participate in the secretomotor and sensory alterations observed in these patients.We determined if a state antibiotics-induced intestinal dysbiosis is able to modify colonic pain-related and motor responses and characterized the neuro-immune mechanisms implicated in mice. A 2-week antibiotics treatment induced a colonic dysbiosis (increments in Bacteroides spp, Clostridium coccoides and Lactobacillus spp and reduction in Bifidobacterium spp). Bacterial adherence was not affected. Dysbiosis was associated with increased levels of secretory-IgA, up-regulation of the antimicrobial lectin RegIIIγ, and toll-like receptors (TLR) 4 and 7 and down-regulation of the antimicrobial-peptide Resistin-Like Molecule-β and TLR5. Dysbiotic mice showed less goblet cells, without changes in the thickness of the mucus layer. Neither macroscopical nor microscopical signs of inflammation were observed. In dysbiotic mice, expression of the cannabinoid receptor 2 was up-regulated, while the cannabinoid 1 and the mu-opioid receptors were down-regulated. In antibiotic-treated mice, visceral pain-related responses elicited by intraperitoneal acetic acid or intracolonic capsaicin were significantly attenuated. Colonic contractility was enhanced during dysbiosis. Intestinal dysbiosis induce changes in the innate intestinal immune system and modulate the expression of pain-related sensory systems, an effect associated with a reduction in visceral pain-related responses. Commensal microbiota modulates gut neuro-immune sensory systems, leading to functional changes, at least as it relates to viscerosensitivity. Similar mechanisms might explain the beneficial effects of antibiotics or certain probiotics in the treatment of IBS.
Keywords: AMP, antimicrobial peptide; CB1/2, cannabinoid receptor type 1 or 2; FGD, functional gastrointestinal disorder; FISH, fluorescent in situ hybridization; GCM, gut commensal microbiota; GI, gastrointestinal; IBS, irritable bowel syndrome; MOR, mu-opioid receptor; NGF, nerve growth factor; PPR, pattern recognition receptor; RELMβ, resistin-like molecule-β; RT-qPCR, reverse transcription quantitative polymerase chain reaction; Reg3γ, regenerating islet-derived protein 3 gamma; SFB, segmented filamentous bacteria; TLR, toll-like receptor; TPH 1/2, tryptophan hydroxylase isoforms 1 or 2; TRPV1/3, transient receptor potential vanilloid types 1 or 3; cannabinoid receptors; colonic motility; gut commensal microbiota; iNOS, inducible nitric oxide synthase; innate immune system; intestinal dysbiosis; opioid receptors; sIgA, secretory IgA; visceral sensitivity.
Figures

References
-
- Sood R, Law GR, Ford AC. Diagnosis of IBS: symptoms, symptom-based criteria, biomarkers or “psychomarkers”? Nat Rev Gastroenterol Hepatol 2014; 11(11):683-91; http://dx.doi.org/10.1038/nrgastro.2014.127 - DOI - PubMed
-
- Simrén M. IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut 2014; 63:1685-6; PMID:24569059; http://dx.doi.org/10.1136/gutjnl-2013-306434 - DOI - PubMed
-
- Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol 2014; 20:2456-69; PMID:24627583; http://dx.doi.org/10.3748/wjg.v20.i10.2456 - DOI - PMC - PubMed
-
- Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K, et al. . Pathogenesis of irritable bowel syndrome-review regarding associated infection and immune activation. Digestion 2013; 87:204-11; PMID:23712295; http://dx.doi.org/10.1159/000350054 - DOI - PubMed
-
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688-93; PMID:16819463;http://dx.doi.org/10.1038/sj.embor.7400731 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
