IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells
- PMID: 25531830
- PMCID: PMC4297567
- DOI: 10.1038/ni.3078
IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential 'inflammatory' type 2 innate lymphoid cells
Abstract
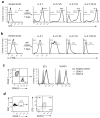
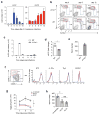
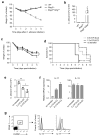
Innate lymphoid cells (ILCs) are lymphocyte-like cells that lack T cell or B cell antigen receptors and mediate protective and repair functions through cytokine secretion. Among these, type 2 ILCs (ILC2 cells) are able to produce type 2 cytokines. We report the existence of an inflammatory ILC2 (iILC2) population responsive to interleukin 25 (IL-25) that complemented IL-33-responsive natural ILC2 (nILC2) cells. iILC2 cells developed into nILC2-like cells in vitro and in vivo and contributed to the expulsion of Nippostrongylus brasiliensis. They also acquired IL-17-producing ability and provided partial protection against Candida albicans. We propose that iILC2 cells are transient progenitors of ILCs mobilized by inflammation and infection that develop into nILC2-like cells or ILC3-like cells and contribute to immunity to both helminths and fungi.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Comment in
-
Inflammatory ILC2 cells: disguising themselves as progenitors?Nat Immunol. 2015 Feb;16(2):133-4. doi: 10.1038/ni.3080. Nat Immunol. 2015. PMID: 25594455 No abstract available.
References
-
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. - PubMed
-
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. - PubMed
-
- Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. - PubMed
-
- Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

