Collagen structure and mechanical properties of the human sclera: analysis for the effects of age
- PMID: 25531905
- PMCID: PMC4340195
- DOI: 10.1115/1.4029430
Collagen structure and mechanical properties of the human sclera: analysis for the effects of age
Abstract
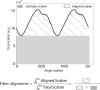
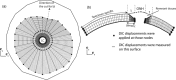
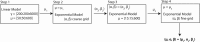
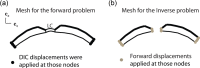
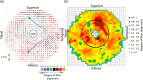
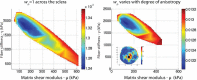
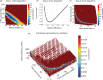
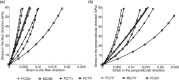
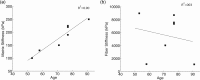
The objective of this study was to measure the collagen fiber structure and estimate the material properties of 7 human donor scleras, from age 53 to 91. The specimens were subjected to inflation testing, and the full-field displacement maps were measured by digital image correlation. After testing, the collagen fiber structure was mapped using wide-angle X-ray scattering. A specimen-specific inverse finite element method was applied to calculate the material properties of the collagen fibers and interfiber matrix by minimizing the difference between the experimental displacements and model predictions. Age effects on the fiber structure and material properties were estimated using multivariate models accounting for spatial autocorrelation. Older age was associated with a larger matrix stiffness (p = 0.001), a lower degree of fiber alignment in the peripapillary sclera (p = 0.01), and a lower mechanical anisotropy in the peripapillary sclera (p = 0.03).
Figures




References
-
- Komai, Y. , and Ushiki, T. , 1991, “The Three-Dimensional Organization of Collagen Fibrils in the Human Cornea and Sclera,” Invest. Ophthalmol. Visual Sci., 32(8), pp. 2244–2258. - PubMed
-
- Kokott, W. , 1934, “Das spaltlinienbild der sklera. (ein beitrag zum funktionellen bau der sklera),” Klin. Monatsbl. Augenheilkd, 92, pp. 177–185.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

