Use of echocardiography reveals reestablishment of ventricular pumping efficiency and partial ventricular wall motion recovery upon ventricular cryoinjury in the zebrafish
- PMID: 25532015
- PMCID: PMC4274112
- DOI: 10.1371/journal.pone.0115604
Use of echocardiography reveals reestablishment of ventricular pumping efficiency and partial ventricular wall motion recovery upon ventricular cryoinjury in the zebrafish
Abstract
Aims: While zebrafish embryos are amenable to in vivo imaging, allowing the study of morphogenetic processes during development, intravital imaging of adults is hampered by their small size and loss of transparency. The use of adult zebrafish as a vertebrate model of cardiac disease and regeneration is increasing at high speed. It is therefore of great importance to establish appropriate and robust methods to measure cardiac function parameters.
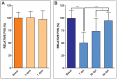
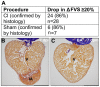
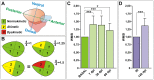
Methods and results: Here we describe the use of 2D-echocardiography to study the fractional volume shortening and segmental wall motion of the ventricle. Our data show that 2D-echocardiography can be used to evaluate cardiac injury and also to study recovery of cardiac function. Interestingly, our results show that while global systolic function recovered following cardiac cryoinjury, ventricular wall motion was only partially restored.
Conclusion: Cryoinjury leads to long-lasting impairment of cardiac contraction, partially mimicking the consequences of myocardial infarction in humans. Functional assessment of heart regeneration by echocardiography allows a deeper understanding of the mechanisms of cardiac regeneration and has the advantage of being easily transferable to other cardiovascular zebrafish disease models.
Conflict of interest statement
Figures


Similar articles
-
Cardiac regeneration following cryoinjury in the adult zebrafish targets a maturation-specific biomechanical remodeling program.Sci Rep. 2018 Oct 23;8(1):15661. doi: 10.1038/s41598-018-33994-8. Sci Rep. 2018. PMID: 30353076 Free PMC article.
-
Advanced echocardiography in adult zebrafish reveals delayed recovery of heart function after myocardial cryoinjury.PLoS One. 2015 Apr 8;10(4):e0122665. doi: 10.1371/journal.pone.0122665. eCollection 2015. PLoS One. 2015. PMID: 25853735 Free PMC article.
-
Induction of myocardial infarction in adult zebrafish using cryoinjury.J Vis Exp. 2012 Apr 18;(62):3666. doi: 10.3791/3666. J Vis Exp. 2012. PMID: 22546770 Free PMC article.
-
Two dimensional echocardiography and Doppler in the right ventricular infarction.Rev Port Cardiol. 1990 Mar;9(3):227-44. Rev Port Cardiol. 1990. PMID: 2202344 Review.
-
[Asynchrony of ventricular contraction and relaxation--pathophysiologically recognized phenomenon, now can be clinically assessed].Herz. 1998 Dec;23(8):506-15. doi: 10.1007/BF03043758. Herz. 1998. PMID: 10023585 Review. German.
Cited by
-
Adaptation of a Mice Doppler Echocardiography Platform to Measure Cardiac Flow Velocities for Embryonic Chicken and Adult Zebrafish.Front Bioeng Biotechnol. 2019 May 14;7:96. doi: 10.3389/fbioe.2019.00096. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31139625 Free PMC article.
-
Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish.Dev Cell. 2018 Feb 26;44(4):433-446.e7. doi: 10.1016/j.devcel.2018.01.021. Dev Cell. 2018. PMID: 29486195 Free PMC article.
-
Cardiac regeneration following cryoinjury in the adult zebrafish targets a maturation-specific biomechanical remodeling program.Sci Rep. 2018 Oct 23;8(1):15661. doi: 10.1038/s41598-018-33994-8. Sci Rep. 2018. PMID: 30353076 Free PMC article.
-
Cell migration during heart regeneration in zebrafish.Dev Dyn. 2016 Jul;245(7):774-87. doi: 10.1002/dvdy.24411. Epub 2016 May 10. Dev Dyn. 2016. PMID: 27085002 Free PMC article. Review.
-
Multiple cryoinjuries modulate the efficiency of zebrafish heart regeneration.Sci Rep. 2020 Jul 14;10(1):11551. doi: 10.1038/s41598-020-68200-1. Sci Rep. 2020. PMID: 32665622 Free PMC article.
References
-
- Jennings RB, Murry CE, Steenbergen C Jr, Reimer KA (1990) Development of cell injury in sustained acute ischemia. Circulation 82:II2–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

