Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex
- PMID: 25532020
- PMCID: PMC4321117
- DOI: 10.1115/1.4029431
Decreased elastic energy storage, not increased material stiffness, characterizes central artery dysfunction in fibulin-5 deficiency independent of sex
Abstract
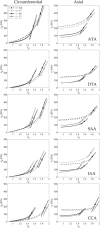
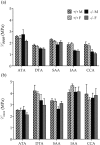
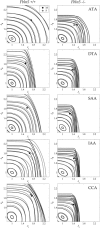
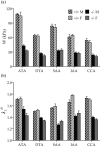
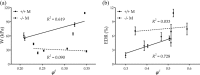
Central artery stiffness has emerged over the past 15 years as a clinically significant indicator of cardiovascular function and initiator of disease. Loss of elastic fiber integrity is one of the primary contributors to increased arterial stiffening in aging, hypertension, and related conditions. Elastic fibers consist of an elastin core and multiple glycoproteins; hence defects in any of these constituents can adversely affect arterial wall mechanics. In this paper, we focus on mechanical consequences of the loss of fibulin-5, an elastin-associated glycoprotein involved in elastogenesis. Specifically, we compared the biaxial mechanical properties of five central arteries-the ascending thoracic aorta, descending thoracic aorta, suprarenal abdominal aorta, infrarenal abdominal aorta, and common carotid artery-from male and female wild-type and fibulin-5 deficient mice. Results revealed that, independent of sex, all five regions in the fibulin-5 deficient mice manifested a marked increase in structural stiffness but also a marked decrease in elastic energy storage and typically an increase in energy dissipation, with all differences being most dramatic in the ascending and abdominal aortas. Given that the primary function of large arteries is to store elastic energy during systole and to use this energy during diastole to work on the blood, fibulin-5 deficiency results in a widespread diminishment of central artery function that can have significant effects on hemodynamics and cardiac function.
Figures


Similar articles
-
Compromised mechanical homeostasis in arterial aging and associated cardiovascular consequences.Biomech Model Mechanobiol. 2018 Oct;17(5):1281-1295. doi: 10.1007/s10237-018-1026-7. Epub 2018 May 12. Biomech Model Mechanobiol. 2018. PMID: 29754316 Free PMC article.
-
Compromised Cardiopulmonary Function in Fibulin-5 Deficient Mice.J Biomech Eng. 2022 Aug 1;144(8):081008. doi: 10.1115/1.4053873. J Biomech Eng. 2022. PMID: 35171214 Free PMC article.
-
Dysfunction in elastic fiber formation in fibulin-5 null mice abrogates the evolution in mechanical response of carotid arteries during maturation.Am J Physiol Heart Circ Physiol. 2013 Mar 1;304(5):H674-86. doi: 10.1152/ajpheart.00459.2012. Epub 2012 Dec 15. Am J Physiol Heart Circ Physiol. 2013. PMID: 23241326 Free PMC article.
-
Fibulin-4 and fibulin-5 in elastogenesis and beyond: Insights from mouse and human studies.Matrix Biol. 2014 Jul;37:142-9. doi: 10.1016/j.matbio.2014.02.004. Epub 2014 Mar 6. Matrix Biol. 2014. PMID: 24613575 Free PMC article. Review.
-
Elastic tissue disruption is a major pathogenic factor to human vascular disease.Mol Biol Rep. 2021 May;48(5):4865-4878. doi: 10.1007/s11033-021-06478-8. Epub 2021 Jun 15. Mol Biol Rep. 2021. PMID: 34129188 Review.
Cited by
-
New insights into arterial stiffening: does sex matter?Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1073-H1087. doi: 10.1152/ajpheart.00132.2018. Epub 2018 Jul 20. Am J Physiol Heart Circ Physiol. 2018. PMID: 30028199 Free PMC article. Review.
-
Mechanisms of Hypoxia-Induced Pulmonary Arterial Stiffening in Mice Revealed by a Functional Genetics Assay of Structural, Functional, and Transcriptomic Data.Front Physiol. 2021 Sep 14;12:726253. doi: 10.3389/fphys.2021.726253. eCollection 2021. Front Physiol. 2021. PMID: 34594238 Free PMC article.
-
Effects of Increased Arterial Stiffness on Atherosclerotic Plaque Amounts.J Biomech Eng. 2018 May 1;140(5):0510071-05100710. doi: 10.1115/1.4039175. J Biomech Eng. 2018. PMID: 29392300 Free PMC article.
-
Sex-dependent differences in central artery haemodynamics in normal and fibulin-5 deficient mice: implications for ageing.Proc Math Phys Eng Sci. 2019 Jan;475(2221):20180076. doi: 10.1098/rspa.2018.0076. Epub 2019 Jan 9. Proc Math Phys Eng Sci. 2019. PMID: 30760948 Free PMC article.
-
Biomechanical and transcriptional evidence that smooth muscle cell death drives an osteochondrogenic phenotype and severe proximal vascular disease in progeria.Biomech Model Mechanobiol. 2023 Aug;22(4):1333-1347. doi: 10.1007/s10237-023-01722-5. Epub 2023 May 7. Biomech Model Mechanobiol. 2023. PMID: 37149823 Free PMC article.
References
-
- Huang, J. , Davis, E. C. , Chapman, S. L. , Budatha, M. , Marmorstein, L. Y. , Word, R. A. , and Yanagisawa, H. , 2010, “Fibulin-4 Deficiency Results in Ascending Aortic Aneurysms: A Potential Link Between Abnormal Smooth Muscle Cell Phenotype and Aneurysm Progression,” Circ. Res., 106(3), pp. 583–592.10.1161/CIRCRESAHA.109.207852 - DOI - PMC - PubMed
-
- Nakamura, T. , Lozano, P. R. , Ikeda, Y. , Iwanaga, Y. , Hinek, A. , Minamisawa, S. , Cheng, C.-F. , Kobuke, K. , Dalton, N. , Takada, Y. , Tashiro, K. , Ross, J., Jr. , Honjo, T. , and Chien, K. R. , 2002, “Fibulin-5/DANCE is Essential for Elastogenesis In Vivo,” Nature, 415(6868), pp. 171–175.10.1038/415171a - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

