Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo
- PMID: 25532138
- PMCID: PMC4933203
- DOI: 10.1038/nmeth.3217
Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo
Erratum in
- Nat Methods. 2015 Jul;12(7):692
Abstract
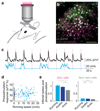
We describe an all-optical strategy for simultaneously manipulating and recording the activity of multiple neurons with cellular resolution in vivo. We performed simultaneous two-photon optogenetic activation and calcium imaging by coexpression of a red-shifted opsin and a genetically encoded calcium indicator. A spatial light modulator allows tens of user-selected neurons to be targeted for spatiotemporally precise concurrent optogenetic activation, while simultaneous fast calcium imaging provides high-resolution network-wide readout of the manipulation with negligible optical cross-talk. Proof-of-principle experiments in mouse barrel cortex demonstrate interrogation of the same neuronal population during different behavioral states and targeting of neuronal ensembles based on their functional signature. This approach extends the optogenetic toolkit beyond the specificity obtained with genetic or viral approaches, enabling high-throughput, flexible and long-term optical interrogation of functionally defined neural circuits with single-cell and single-spike resolution in the mouse brain in vivo.
Conflict of interest statement
All authors declare that there are no competing financial interests
Figures



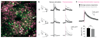
References
-
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

