Synthetic Biology: A Bridge between Artificial and Natural Cells
- PMID: 25532531
- PMCID: PMC4284483
- DOI: 10.3390/life4041092
Synthetic Biology: A Bridge between Artificial and Natural Cells
Abstract
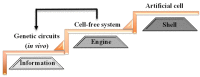
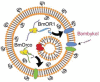
Artificial cells are simple cell-like entities that possess certain properties of natural cells. In general, artificial cells are constructed using three parts: (1) biological membranes that serve as protective barriers, while allowing communication between the cells and the environment; (2) transcription and translation machinery that synthesize proteins based on genetic sequences; and (3) genetic modules that control the dynamics of the whole cell. Artificial cells are minimal and well-defined systems that can be more easily engineered and controlled when compared to natural cells. Artificial cells can be used as biomimetic systems to study and understand natural dynamics of cells with minimal interference from cellular complexity. However, there remain significant gaps between artificial and natural cells. How much information can we encode into artificial cells? What is the minimal number of factors that are necessary to achieve robust functioning of artificial cells? Can artificial cells communicate with their environments efficiently? Can artificial cells replicate, divide or even evolve? Here, we review synthetic biological methods that could shrink the gaps between artificial and natural cells. The closure of these gaps will lead to advancement in synthetic biology, cellular biology and biomedical applications.
Figures


References
-
-
Hooke, R. Micrographia: Or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses With Observations and Inquiries Thereupon; John Martyn, printer to the Royal Society, and are to be sold at his shop at the Bell a little without Temple Barr. Martin and Allestry, London, UK, 1665.
-
-
- Schwann T. Mikroskopische untersuchungen über die übereinstimmung in der struktur und dem wachstum der tiere und pflanzen. In: Jahn I., editor. Klassische Schriften zur Zellenlehre. 2nd ed. Wissenschaftlicher Verlag Harri Deutsch GmbH; Frankfurt, Germany: 2003.
-
- Chang T.M. 1957 Report on “method for preparing artificial hemoglobin corpuscles”. [(accessed on 11 December 2014)]. Available online: http://www.worldscientific.com/doi/pdf/10.1142/9789812770370_bmatter. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

