Tumor mechanics and metabolic dysfunction
- PMID: 25532934
- PMCID: PMC4339308
- DOI: 10.1016/j.freeradbiomed.2014.11.020
Tumor mechanics and metabolic dysfunction
Abstract
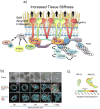
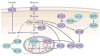
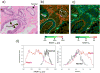
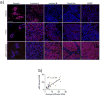
Desmosplasia is a characteristic of most solid tumors and leads to fibrosis through abnormal extracellular matrix (ECM) deposition, remodeling, and posttranslational modifications. The resulting stiff tumor stroma not only compromises vascular integrity to induce hypoxia and impede drug delivery, but also promotes aggressiveness by potentiating the activity of key growth, invasion, and survival pathways. Intriguingly, many of the protumorigenic signaling pathways that are mechanically activated by ECM stiffness also promote glucose uptake and aerobic glycolysis, and an altered metabolism is a recognized hallmark of cancer. Indeed, emerging evidence suggests that metabolic alterations and an abnormal ECM may cooperatively drive cancer cell aggression and treatment resistance. Accordingly, improved methods to monitor tissue mechanics and metabolism promise to improve diagnostics and treatments to ameliorate ECM stiffening and elevated mechanosignaling may improve patient outcome. Here we discuss the interplay between ECM mechanics and metabolism in tumor biology and suggest that monitoring these processes and targeting their regulatory pathways may improve diagnostics, therapy, and the prevention of malignant transformation.
Keywords: Cancer; ECM stiffness; Free radicals; Mechanosignaling; Tumor metabolism; Tumor microenvironment.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Ai B, Pan T, et al. The relationship of expression of GLUT1,HIF-1alpha and the uptake of FDG in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2007;10(6):508–512. - PubMed
-
- Bae YS, Kang SW, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272(1):217–221. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA114462/CA/NCI NIH HHS/United States
- R01 CA169175/CA/NCI NIH HHS/United States
- U01ES019458/ES/NIEHS NIH HHS/United States
- R01CA158668/CA/NCI NIH HHS/United States
- U54CA143836/CA/NCI NIH HHS/United States
- R01 CA170851/CA/NCI NIH HHS/United States
- R01 CA136590/CA/NCI NIH HHS/United States
- R01 CA158668/CA/NCI NIH HHS/United States
- R01 CA138818/CA/NCI NIH HHS/United States
- F32 CA174319/CA/NCI NIH HHS/United States
- R33CA183685-01/CA/NCI NIH HHS/United States
- R01CA170851/CA/NCI NIH HHS/United States
- T32 HL007544/HL/NHLBI NIH HHS/United States
- CA138818-01A1/CA/NCI NIH HHS/United States
- T32HL007544/HL/NHLBI NIH HHS/United States
- R01 CA155664/CA/NCI NIH HHS/United States
- F32CA174319/CA/NCI NIH HHS/United States
- R01CA114462/CA/NCI NIH HHS/United States
- R01 CA174929/CA/NCI NIH HHS/United States
- R01CA142833/CA/NCI NIH HHS/United States
- U54 CA163155/CA/NCI NIH HHS/United States
- U01 ES019458/ES/NIEHS NIH HHS/United States
- R01 CA192914/CA/NCI NIH HHS/United States
- U54CA163155-01/CA/NCI NIH HHS/United States
- R33 CA183685/CA/NCI NIH HHS/United States
- R21EB008811/EB/NIBIB NIH HHS/United States
- R21 EB008811/EB/NIBIB NIH HHS/United States
- R01 CA142833/CA/NCI NIH HHS/United States
- U54 CA143836/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

