Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma
- PMID: 25533035
- PMCID: PMC4335079
- DOI: 10.1182/blood-2014-08-595801
Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma
Abstract
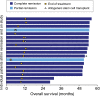
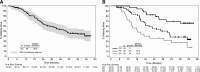
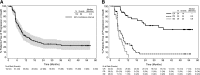
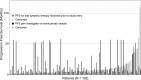
We present response and survival outcomes of a pivotal phase 2 trial of the antibody-drug conjugate brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma following autologous stem cell transplant (N = 102) after a median observation period of approximately 3 years. Median overall survival and progression-free survival were estimated at 40.5 months and 9.3 months, respectively. Improved outcomes were observed in patients who achieved a complete remission (CR) on brentuximab vedotin, with estimated 3-year overall survival and progression-free survival rates of 73% (95% confidence interval [CI]: 57%, 88%) and 58% (95% CI: 41%, 76%), respectively, in this group (medians not reached). Of the 34 patients who obtained CR, 16 (47%) remain progression-free after a median of 53.3 months (range, 29.0 to 56.2 months) of observation; 12 patients remain progression-free without a consolidative allogeneic stem cell transplant. Younger age, good performance status, and lower disease burden at baseline were characteristic of patients who achieved a CR and were favorable prognostic factors for overall survival. These results suggest that a significant proportion of patients who respond to brentuximab vedotin can achieve prolonged disease control. The trial was registered at www.clinicaltrials.gov as #NCT00848926.
© 2015 by The American Society of Hematology.
Figures
References
-
- Sureda A, Arranz R, Iriondo A, et al. Grupo Español de Linformas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autólogo de Médula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19(5):1395–1404. - PubMed
-
- Sureda A, Constans M, Iriondo A, et al. Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea Cooperative Group. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. - PubMed
-
- Arai S, Fanale M, DeVos S, et al. Defining a Hodgkin lymphoma population for novel therapeutics after relapse from autologous hematopoietic cell transplant. Leuk Lymphoma. 2013;54(11):2531–2533. - PubMed
-
- Crump M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology (Am Soc Hematol Educ Program) 2008:326–333. - PubMed
-
- Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study: a prospective clinical trial by the Grupo Español de Linfomas/Trasplante de Médula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–317. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

