Indirect comparisons of ranibizumab and dexamethasone in macular oedema secondary to retinal vein occlusion
- PMID: 25533265
- PMCID: PMC4289570
- DOI: 10.1186/1471-2288-14-140
Indirect comparisons of ranibizumab and dexamethasone in macular oedema secondary to retinal vein occlusion
Abstract
Background: Two treatments, ranibizumab and dexamethasone implant, for visual impairment due to macular oedema (ME) secondary to retinal vein occlusion (RVO) have recently been studied in clinical trials. There have been no head to head comparisons of the two treatments, and improvement measured as gain in Best Corrected Visual Acuity (BCVA) was reported using different outcomes thresholds between trials. To overcome these limitations, and inform an economic model, we developed a combination of a multinomial model and an indirect Bayesian comparison model for multinomial outcomes.
Methods: Outcomes of change from baseline in BCVA for dexamethasone compatible with those available for ranibizumab, reported by 4 randomised controlled trials, were estimated by fitting a multinomial distribution model to the probability of a patient achieving outcomes in a range of changes from baseline in BCVA (numbers of letters) at month 1. A Bayesian indirect comparison multinomial model was then developed to compare treatments in the Branch RVO (BRVO) and Central RVO (CRVO) populations.
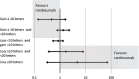
Results: The multinomial model had excellent fit to the observed results. With the Bayesian indirect comparison, the probabilities of achieving ≥20 letters, with 95% credible intervals, at month 1 in patients with BRVO were 0.191 (0.130, 0.261) with ranibizumab and 0.093 (0.027, 0.213) with dexamethasone. In patients with CRVO, probabilities were 0.133 (0.082, 0.195) (ranibizumab) and 0.063 (0.016, 0.153) (dexamethasone). Probabilities of a gain in ≥10 letters in BRVO patients were 0.500 (0.365, 0.650) v 0.459 (0.248, 0.724) and in CRVO patients 0.459 (0.332, 0.602) v 0.498 (0.263, 0.791) for ranibizumab and dexamethasone treatments respectively. The comparisons also favoured ranibizumab at month 6 although changes to therapies after month 3 may have introduced bias.
Conclusion: The newly developed combination of multinomial and indirect Bayesian comparison models indicated a trend for ranibizumab association with a greater percentage of ME patients achieving visual gains than dexamethasone at months 1 and 6 in a common clinical context, although results were not classically significant. The method was a useful tool for comparisons of probability distributions between clinical trials that reported events on different categorical scales and estimates can be used to inform economic models.
Figures



References
-
- Group SSR. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 6. Arch Ophthalmol. 2009;127:1115–1128. doi: 10.1001/archophthalmol.2009.233. - DOI - PMC - PubMed
Pre-publication history
-
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2288/14/140/prepub
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

