K+ congeners that do not compromise Na+ activation of the Na+,K+-ATPase: hydration of the ion binding cavity likely controls ion selectivity
- PMID: 25533461
- PMCID: PMC4319036
- DOI: 10.1074/jbc.M114.577486
K+ congeners that do not compromise Na+ activation of the Na+,K+-ATPase: hydration of the ion binding cavity likely controls ion selectivity
Abstract
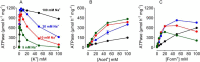
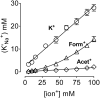
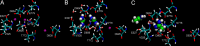
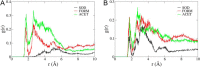
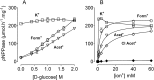
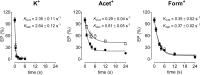
The Na(+),K(+)-ATPase is essential for ionic homeostasis in animal cells. The dephosphoenzyme contains Na(+) selective inward facing sites, whereas the phosphoenzyme contains K(+) selective outward facing sites. Under normal physiological conditions, K(+) inhibits cytoplasmic Na(+) activation of the enzyme. Acetamidinium (Acet(+)) and formamidinium (Form(+)) have been shown to permeate the pump through the outward facing sites. Here, we show that these cations, unlike K(+), are unable to enter the inward facing sites in the dephosphorylated enzyme. Consistently, the organic cations exhibited little to no antagonism to cytoplasmic Na(+) activation. Na(+),K(+)-ATPase structures revealed a previously undescribed rotamer transition of the hydroxymethyl side chain of the absolutely conserved Thr(772) of the α-subunit. The side chain contributes its hydroxyl to Na(+) in site I in the E1 form and rotates to contribute its methyl group toward K(+) in the E2 form. Molecular dynamics simulations to the E1·AlF4 (-)·ADP·3Na(+) structure indicated that 1) bound organic cations differentially distorted the ion binding sites, 2) the hydroxymethyl of Thr(772) rotates to stabilize bound Form(+) through water molecules, and 3) the rotamer transition is mediated by water traffic into the ion binding cavity. Accordingly, dehydration induced by osmotic stress enhanced the interaction of the congeners with the outward facing sites and profoundly modified the organization of membrane domains of the α-subunit. These results assign a catalytic role for water in pump function, and shed light on a backbone-independent but a conformation-dependent switch between H-bond and dispersion contact as part of the catalytic mechanism of the Na(+),K(+)-ATPase.
Keywords: ATPase; Membrane Enzyme; Membrane Protein; Na+/K+-ATPase; Potassium Transport.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Kühlbrandt W. (2004) Biology, structure, and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 5, 282–295 - PubMed
-
- Kaplan J. H. (2002) Biochemistry of the Na+,K+-ATPase. Annu. Rev. Biochem. 71, 511–535 - PubMed
-
- Geering K. (2008) Functional roles of Na+,K+-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 17, 526–532 - PubMed
-
- Jorgensen P. L., Pedersen P. A. (2001) Structure-function relationships of Na+, K+, ATP, or Mg2+ binding and energy transduction in Na+,K+-ATPase. Biochim. Biophys. Acta 1505, 57–74 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

