A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2
- PMID: 25533462
- PMCID: PMC4319031
- DOI: 10.1074/jbc.M114.590067
A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2
Abstract
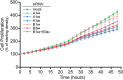
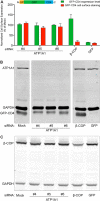
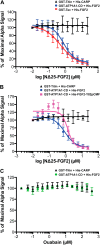
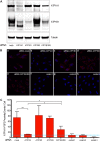
Previous studies proposed a role for the Na/K-ATPase in unconventional secretion of fibroblast growth factor 2 (FGF2). This conclusion was based upon pharmacological inhibition of FGF2 secretion in the presence of ouabain. However, neither independent experimental evidence nor a potential mechanism was provided. Based upon an unbiased RNAi screen, we now report the identification of ATP1A1, the α1-chain of the Na/K-ATPase, as a factor required for efficient secretion of FGF2. As opposed to ATP1A1, down-regulation of the β1- and β3-chains (ATP1B1 and ATP1B3) of the Na/K-ATPase did not affect FGF2 secretion, suggesting that they are dispensable for this process. These findings indicate that it is not the membrane potential-generating function of the Na/K-ATPase complex but rather a so far unidentified role of potentially unassembled α1-chains that is critical for unconventional secretion of FGF2. Consistently, in the absence of β-chains, we found a direct interaction between the cytoplasmic domain of ATP1A1 and FGF2 with submicromolar affinity. Based upon these observations, we propose that ATP1A1 is a recruitment factor for FGF2 at the inner leaflet of plasma membranes that may control phosphatidylinositol 4,5-bisphosphate-dependent membrane translocation as part of the unconventional secretory pathway of FGF2.
Keywords: ATP1A1; Fibroblast Growth Factor (FGF); Heparan Sulfate; Membrane Recruitment; Membrane Translocation; Na+/K+-ATPase; Phosphoinositide; Plasma Membrane; Tec Kinase; Unconventional Secretion.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Rothman J. E. (1994) Mechanisms of intracellular protein transport. Nature 372, 55–63 - PubMed
-
- Rothman J. E., Wieland F. T. (1996) Protein sorting by transport vesicles. Science 272, 227–234 - PubMed
-
- Schekman R., Orci L. (1996) Coat proteins and vesicle budding. Science 271, 1526–1533 - PubMed
-
- Rapoport T. A. (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

