Randomised controlled trial of mesalazine in IBS
- PMID: 25533646
- PMCID: PMC4717362
- DOI: 10.1136/gutjnl-2014-308188
Randomised controlled trial of mesalazine in IBS
Abstract
Objective: Low-grade intestinal inflammation plays a role in the pathophysiology of IBS. In this trial, we aimed at evaluating the efficacy and safety of mesalazine in patients with IBS.
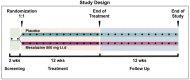
Design: We conducted a phase 3, multicentre, tertiary setting, randomised, double-blind, placebo-controlled trial in patients with Rome III confirmed IBS. Patients were randomly assigned to either mesalazine, 800 mg, or placebo, three times daily for 12 weeks, and were followed for additional 12 weeks. The primary efficacy endpoint was satisfactory relief of abdominal pain/discomfort for at least half of the weeks of the treatment period. The key secondary endpoint was satisfactory relief of overall IBS symptoms. Supportive analyses were also performed classifying as responders patients with a percentage of affirmative answers of at least 75% or >75% of time.
Results: A total of 185 patients with IBS were enrolled from 21 centres. For the primary endpoint, the responder patients were 68.6% in the mesalazine group versus 67.4% in the placebo group (p=0.870; 95% CI -12.8 to 15.1). In explorative analyses, with the 75% rule or >75% rule, the percentage of responders was greater in the mesalazine group with a difference over placebo of 11.6% (p=0.115; 95% CI -2.7% to 26.0%) and 5.9% (p=0.404; 95% CI -7.8% to 19.4%), respectively, although these differences were not significant. For the key secondary endpoint, overall symptoms improved in the mesalazine group and reached a significant difference of 15.1% versus placebo (p=0.032; 95% CI 1.5% to 28.7%) with the >75% rule.
Conclusions: Mesalazine treatment was not superior than placebo on the study primary endpoint. However, a subgroup of patients with IBS showed a sustained therapy response and benefits from a mesalazine therapy.
Trial registration number: ClincialTrials.gov number, NCT00626288.
Keywords: ABDOMINAL PAIN; CLINICAL TRIALS; GUT INFLAMMATION; INFLAMMATORY CELLS; IRRITABLE BOWEL SYNDROME.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures



Comment in
-
Efficacy of mesalazine in IBS.Gut. 2016 Jan;65(1):187-8. doi: 10.1136/gutjnl-2015-309593. Epub 2015 Apr 14. Gut. 2016. PMID: 25873641 No abstract available.
-
In search for a disease-modifying treatment in irritable bowel syndrome.Gut. 2016 Jan;65(1):2-3. doi: 10.1136/gutjnl-2015-310024. Epub 2015 Jun 25. Gut. 2016. PMID: 26113178 No abstract available.
References
-
- Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. - PubMed
-
- Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2013;368:578–9. - PubMed
-
- Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009;136:1979–88. - PubMed
-
- Cremon C, Stanghellini V, Pallotti F, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterology 2014;147:69–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
