A novel patient-derived tumorgraft model with TRAF1-ALK anaplastic large-cell lymphoma translocation
- PMID: 25533804
- PMCID: PMC4864432
- DOI: 10.1038/leu.2014.347
A novel patient-derived tumorgraft model with TRAF1-ALK anaplastic large-cell lymphoma translocation
Abstract
Although anaplastic large-cell lymphomas (ALCL) carrying anaplastic lymphoma kinase (ALK) have a relatively good prognosis, aggressive forms exist. We have identified a novel translocation, causing the fusion of the TRAF1 and ALK genes, in one patient who presented with a leukemic ALK+ ALCL (ALCL-11). To uncover the mechanisms leading to high-grade ALCL, we developed a human patient-derived tumorgraft (hPDT) line. Molecular characterization of primary and PDT cells demonstrated the activation of ALK and nuclear factor kB (NFkB) pathways. Genomic studies of ALCL-11 showed the TP53 loss and the in vivo subclonal expansion of lymphoma cells, lacking PRDM1/Blimp1 and carrying c-MYC gene amplification. The treatment with proteasome inhibitors of TRAF1-ALK cells led to the downregulation of p50/p52 and lymphoma growth inhibition. Moreover, a NFkB gene set classifier stratified ALCL in distinct subsets with different clinical outcome. Although a selective ALK inhibitor (CEP28122) resulted in a significant clinical response of hPDT mice, nevertheless the disease could not be eradicated. These data indicate that the activation of NFkB signaling contributes to the neoplastic phenotype of TRAF1-ALK ALCL. ALCL hPDTs are invaluable tools to validate the role of druggable molecules, predict therapeutic responses and implement patient specific therapies.
Conflict of interest statement
Authors must declare to not have any competing financial interests in relation to the work described.
Figures


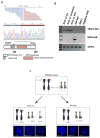




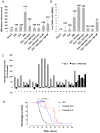
References
-
- Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, et al. WHO classification of tumors of haemotolopoietic and lymphoid tissues. 4. Stylus Publishing, LLC; Sterling, VA: 2008.
-
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504. - PubMed
-
- Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. - PubMed
-
- Monaco S, Tsao L, Murty VV, Nandula SV, Donovan V, Oesterheld J, et al. Pediatric ALK+ anaplastic large cell lymphoma with t(3;8)(q26.2;q24) translocation and c-myc rearrangement terminating in a leukemic phase. Am J Hematol. 2007;82(1):59–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

