Osmotic surveillance mediates rapid wound closure through nucleotide release
- PMID: 25533845
- PMCID: PMC4274268
- DOI: 10.1083/jcb.201408049
Osmotic surveillance mediates rapid wound closure through nucleotide release
Abstract
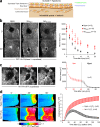
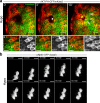
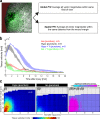
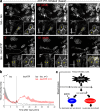
Osmotic cues from the environment mediate rapid detection of epithelial breaches by leukocytes in larval zebrafish tail fins. Using intravital luminescence and fluorescence microscopy, we now show that osmolarity differences between the interstitial fluid and the external environment trigger ATP release at tail fin wounds to initiate rapid wound closure through long-range activation of basal epithelial cell motility. Extracellular nucleotide breakdown, at least in part mediated by ecto-nucleoside triphosphate diphosphohydrolase 3 (Entpd3), restricts the range and duration of osmotically induced cell migration after injury. Thus, in zebrafish larvae, wound repair is driven by an autoregulatory circuit that generates pro-migratory tissue signals as a function of environmental exposure of the inside of the tissue.
© 2014 Gault et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

