A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol
- PMID: 25533949
- PMCID: PMC4368482
- DOI: 10.1038/nature14028
A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol
Abstract
Resveratrol is reported to extend lifespan and provide cardio-neuro-protective, anti-diabetic, and anti-cancer effects by initiating a stress response that induces survival genes. Because human tyrosyl transfer-RNA (tRNA) synthetase (TyrRS) translocates to the nucleus under stress conditions, we considered the possibility that the tyrosine-like phenolic ring of resveratrol might fit into the active site pocket to effect a nuclear role. Here we present a 2.1 Å co-crystal structure of resveratrol bound to the active site of TyrRS. Resveratrol nullifies the catalytic activity and redirects TyrRS to a nuclear function, stimulating NAD(+)-dependent auto-poly-ADP-ribosylation of poly(ADP-ribose) polymerase 1 (PARP1). Downstream activation of key stress signalling pathways are causally connected to TyrRS-PARP1-NAD(+) collaboration. This collaboration is also demonstrated in the mouse, and is specifically blocked in vivo by a resveratrol-displacing tyrosyl adenylate analogue. In contrast to functionally diverse tRNA synthetase catalytic nulls created by alternative splicing events that ablate active sites, here a non-spliced TyrRS catalytic null reveals a new PARP1- and NAD(+)-dependent dimension to the physiological mechanism of resveratrol.
Conflict of interest statement
Although P.S. has a financial interest in aTyr Pharma (who partially supported this investigation), he and M. S. have no financial interest in this specific work. X-ray structure co-ordinates of resveratrol bound TyrRS (PDB ID code 4Q93) and L-Tyr bound TyrRS (PDB ID code 4QBT) were deposited to the Protein Data Bank.
Figures
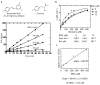
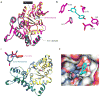
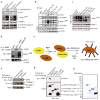

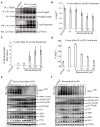

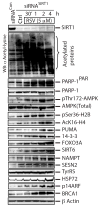


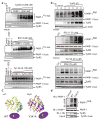


References
-
- Jang M, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

